A kind of method utilizing microchannel reaction device to prepare rifampicin
A technology of channel reaction device and microchannel reaction, which is applied in the direction of organic chemistry, can solve the problems of high price and the impact of cost on income, and achieve the effects of short reaction time, saving raw material costs, low toxicity and pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
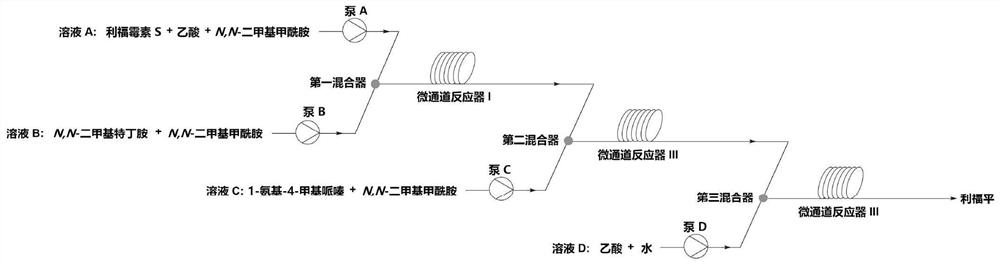
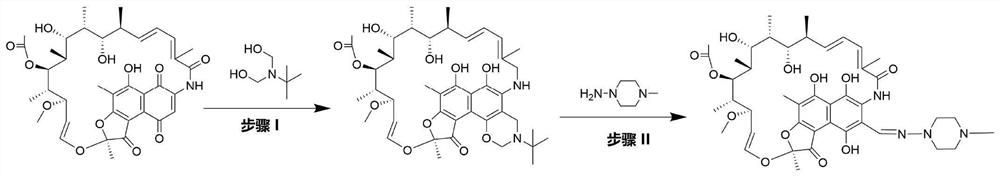
[0038] Get 10g of 97% rifamycin S and 3.75ml of 99.5% by mass concentration of acetic acid and 10ml of 98% of 98% purity and mix it with 25ml of homogeneous liquid A; N, N-dimethylol terbutylamine with a purity of 98% in ml becomes a homogeneous liquid B without adding a solvent; 2.31 ml of 1-amino-4-methylpiperazine with a purity of 98% and 22.5 ml of a purity of 98 % N,N-dimethylformamide mixed into 25ml of homogeneous liquid C; 1L of distilled water was taken as homogeneous liquid D; homogeneous liquid A was extracted by pump A at a flow rate of 0.885mL / min, and homogeneous liquid was extracted by pump B Phase liquid B, the flow rate is 0.115mL / min, the two are fully mixed with an inverted Y-shaped mixing valve, and then pumped into the microchannel reactor I together, the volume of the microchannel reactor is 20ml, the reaction temperature is 75°C, and the reaction time is 20min , the outflowing reaction solution is a mixed liquid of N-tertidine-1,3-oxazine (5,6-C) rifamyc...
Embodiment 2
[0040] Get 10g of rifamycin S with a purity of 97% and 3.75ml of acetic acid with a concentration of 99.5% by mass and 10ml of a purity of 98% N,N-dimethylformamide and mix it with 25ml of homogeneous liquid A; take 3.2 N,N-dimethylol terbutylamine with a purity of 98% in ml and N,N-dimethylformamide with a purity of 97ml are mixed to form 100ml of homogeneous liquid B; Mix 1-amino-4-methylpiperazine with 22.5ml of N,N-dimethylformamide with a purity of 98% to form 25ml of homogeneous liquid C; take 1L of distilled water as homogeneous liquid D; Liquid A, the flow rate is 0.2mL / min, the homogeneous liquid B is extracted by the pump B, the flow rate is 0.8mL / min, the two are fully mixed with an inverted Y-shaped mixing valve, and then pumped into the microchannel reactor I together, the microchannel reaction The volume of the device is 20ml, the reaction temperature is 75°C, and the reaction time is 20min. Formamide mixed liquid, the actual molar ratio of rifamycin S and N,N-d...
Embodiment 3
[0042] Get 10g of rifamycin S with a purity of 97% and 7.5ml of mass percent concentration as 99.5% acetic acid and 14ml of 98% purity N, N-dimethylformamide mixed to be 25ml of homogeneous liquid A; take 2.44 N, N-dimethylol terbutylamine with a purity of 98% in ml becomes a homogeneous liquid B without solvent; 2.12 ml of 1-amino-4-methylpiperazine with a purity of 98% and 22.5 ml of a purity of 98 % N,N-dimethylformamide mixed into 25ml of homogeneous liquid C; 1L of distilled water was taken as homogeneous liquid D; homogeneous liquid A was extracted by pump A at a flow rate of 0.885mL / min, and homogeneous liquid was extracted by pump B Phase liquid B, the flow rate is 0.115mL / min, the two are fully mixed with an inverted Y-shaped mixing valve, and then pumped into the microchannel reactor I together, the volume of the microchannel reactor is 20ml, the reaction temperature is 75°C, and the reaction time is 20min , the outflowing reaction solution is a mixed liquid of N-ter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com