Biomarker for diagnosing mycobacterium tuberculosis infection and related kit
A biomarker, Mycobacterium tuberculosis technology, applied in the field of biological diagnosis, can solve the problems of inability to determine the results, insufficient sensitivity, etc., to achieve the effect of improving the diagnostic efficiency, rapid operation, high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
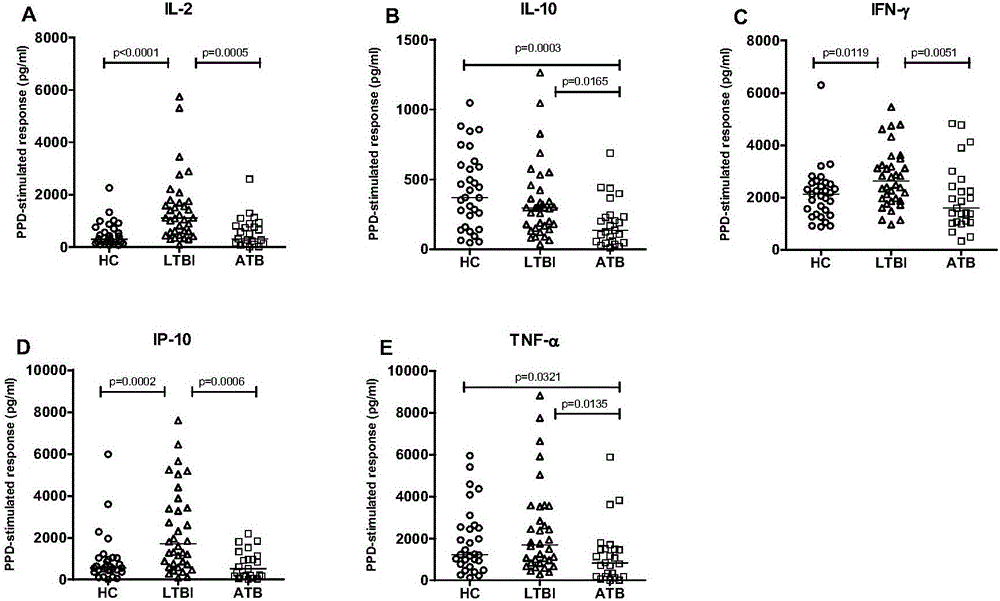
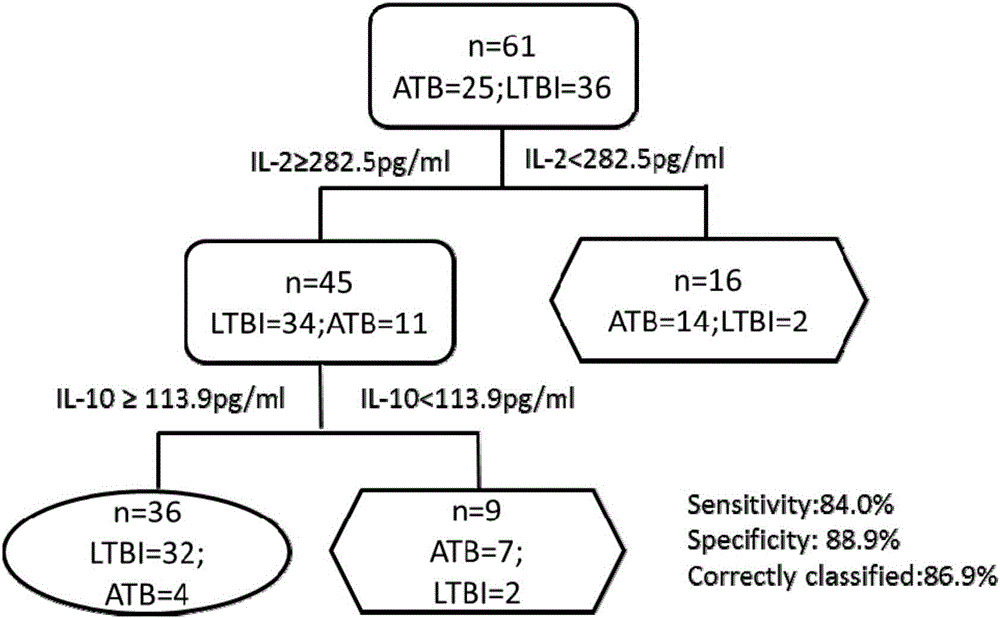
[0031] Example 1: Screening of biomarkers for diagnosis of Mycobacterium tuberculosis infection
[0032] Step 1: Case collection
[0033] Subject samples were collected, including blood samples from patients with active tuberculosis, patients with latent tuberculosis infection and healthy controls. The basis for the diagnosis of active pulmonary tuberculosis in the patient is: the patient has a positive sputum smear result or a positive sputum culture result, abnormal X-ray chest film or less than 30 days of anti-tuberculosis treatment; the inclusion criteria for patients with latent tuberculosis infection are: High-risk factors for tuberculosis infection or close contact with active tuberculosis patients, tuberculosis-specific or The test was positive, and there was no evidence of tuberculosis infection in chest radiographs and sputum smears; healthy controls had no high-risk factors for tuberculosis infection, no recent history of close contact with active tuberculosis, ...
Embodiment 2
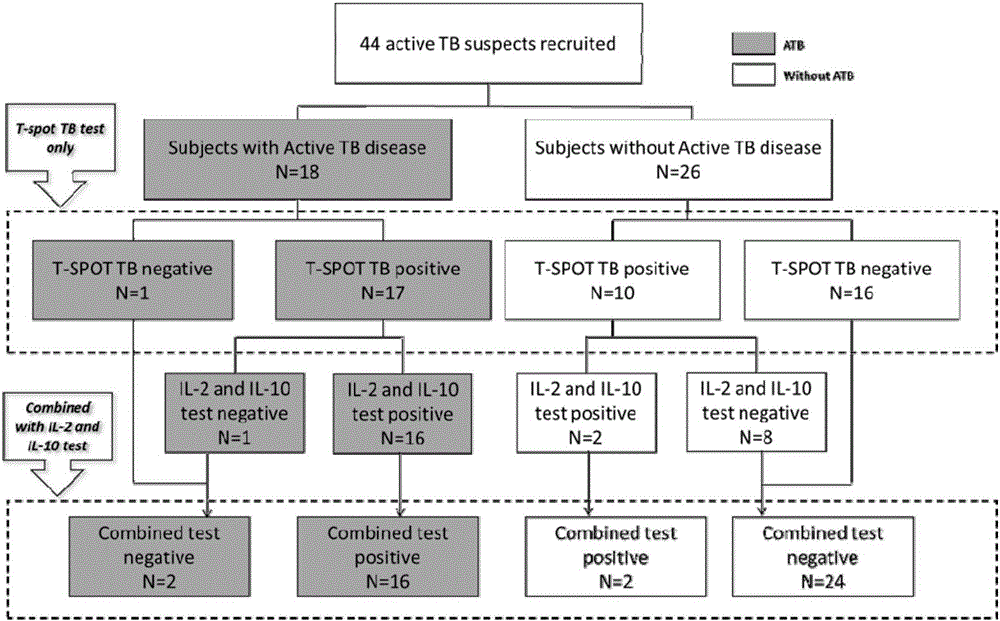
[0059] Example 2: Clinical application of biomarkers for diagnosis of Mycobacterium tuberculosis infection and related kits
[0060] Step 1: Case collection
[0061] Suspected patients with active tuberculosis were collected clinically, and the inclusion criteria were: clinical manifestations similar to active tuberculosis, such as cough, hemoptysis, weight loss, fever, and night sweats, etc. High-risk factors for infection or close contact history of active tuberculosis patients. In this example, according to the above inclusion criteria, a total of 44 suspected active tuberculosis patients were collected.
[0062] Step 2: Specimen Collection and Processing
[0063] Take 5-10ml of venous blood from the collected peripheral blood of suspected patients with active tuberculosis into vacuum blood collection tubes; further separate peripheral blood mononuclear cells: centrifuge the vacuum blood collection tubes at a speed of 2800-3000rpm, and the centrifugation time After the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com