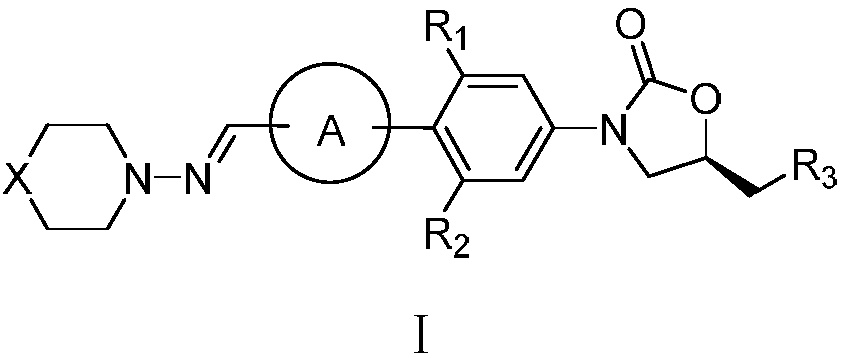
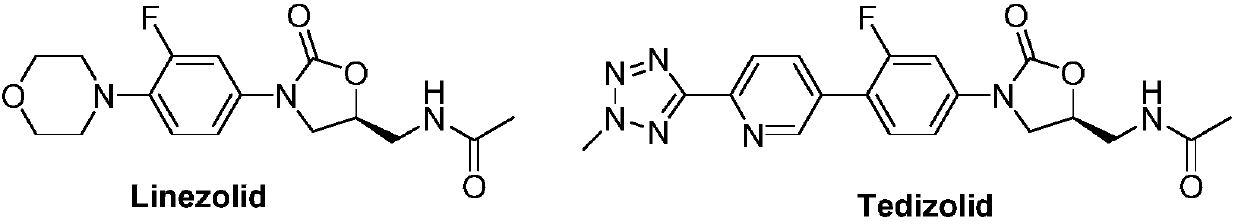
Oxazolidinone compound containing combined aromatic hydrazine and its preparation method
A compound, oxazolidine technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1 (S)-N-(3-{2-fluoro-4'-[(4-methylpiperazin-1-yl)iminomethyl]biphenyl-4-yl}-2-oxo Preparation of (1,3-oxazolidin-5-ylmethyl)acetamide (Ia)
[0109] 1.1(S)-N-[3-(2-fluoro-4'-formylbiphenyl-4-yl)-2-oxo-1,3-oxazolidin-5-ylmethyl]acetamide Synthesis of (L4)
[0110] 1.1.1 Preparation of methyl (3-fluorophenyl) carbamate (L1)
[0111] Under an ice-salt bath, add 44.4g (0.40mol) of m-fluoroaniline and 31.6g (0.40mol) of pyridine into 266mL of dichloromethane, and stir until completely dissolved. Control the temperature at -5~0°C, add 41.4g (0.44mol) of methyl chloroformate in dichloromethane (50mL) dropwise to the reaction liquid, after 1.5h, react at room temperature for 2h. After the reaction, the reaction solution was washed with water (150mL×2), 1M hydrochloric acid (150mL) and saturated brine (150mL) successively, the organic layer was dried over anhydrous sodium sulfate, filtered with suction, the filtrate was concentrated to dryness under reduced pressure, a...
Embodiment 2
[0125] Example 2 (S)-N-{3-[2-fluoro-4'-(morpholin-4-yliminomethyl)biphenyl-4-yl]-2-oxo-1,3- Preparation of oxazolidin-5-ylmethyl}acetamide (Ib)
[0126] Using morpholine as a raw material, synthesize the key intermediate according to the synthesis method of 1.2 in Example 1, and then synthesize (S)-N-{3-[2-fluoro-4' according to the synthesis method of 1.3 in Example 1 -(morpholin-4-yliminomethyl)biphenyl-4-yl]-2-oxo-1,3-oxazolidin-5-ylmethyl}acetamide. Yield 78%. MS(ESI),m / z(%):441.1 [M+H] + ; 1 H-NMR (400MHz, DMSO-d6): δ8.27(t, J=5.7Hz, 1H), 7.74(s, 1H), 7.68(s, 1H), 7.66(s, 1H), 7.59(ddd, J=14.4,10.9,4.7Hz,4H),7.42(dd,J=8.6,2.0Hz,1H),4.77(m,1H),4.17(t,J=9.0Hz,1H),3.79(dd,J =10.7,6.2Hz,5H),3.44(t, J=5.4Hz,2H),3.20–3.07(m,4H),1.85(s,3H).
Embodiment 3
[0127] Example 3 (S)-N-{3-[2-fluoro-4'-(thiomorpholin-4-yliminomethyl)biphenyl-4-yl]-2-oxo-1, Preparation of 3-oxazolidin-5-ylmethyl}acetamide (Id)
[0128] Using thiomorpholine as a raw material, synthesize key intermediates according to the synthetic method of 1.2 in Example 1, and then synthesize (S)-N-{3-[2-fluoro- 4'-(thiomorpholin-4-yliminomethyl)biphenyl-4-yl]-2-oxo-1,3-oxazolidin-5-ylmethyl}acetamide. Yield 80%. MS(ESI),m / z(%):457.5[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com