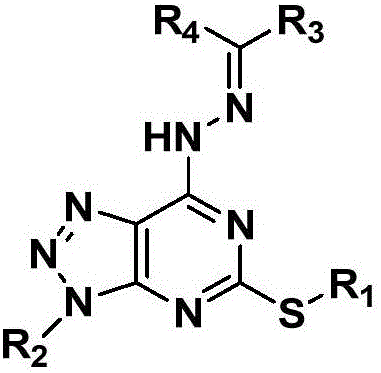
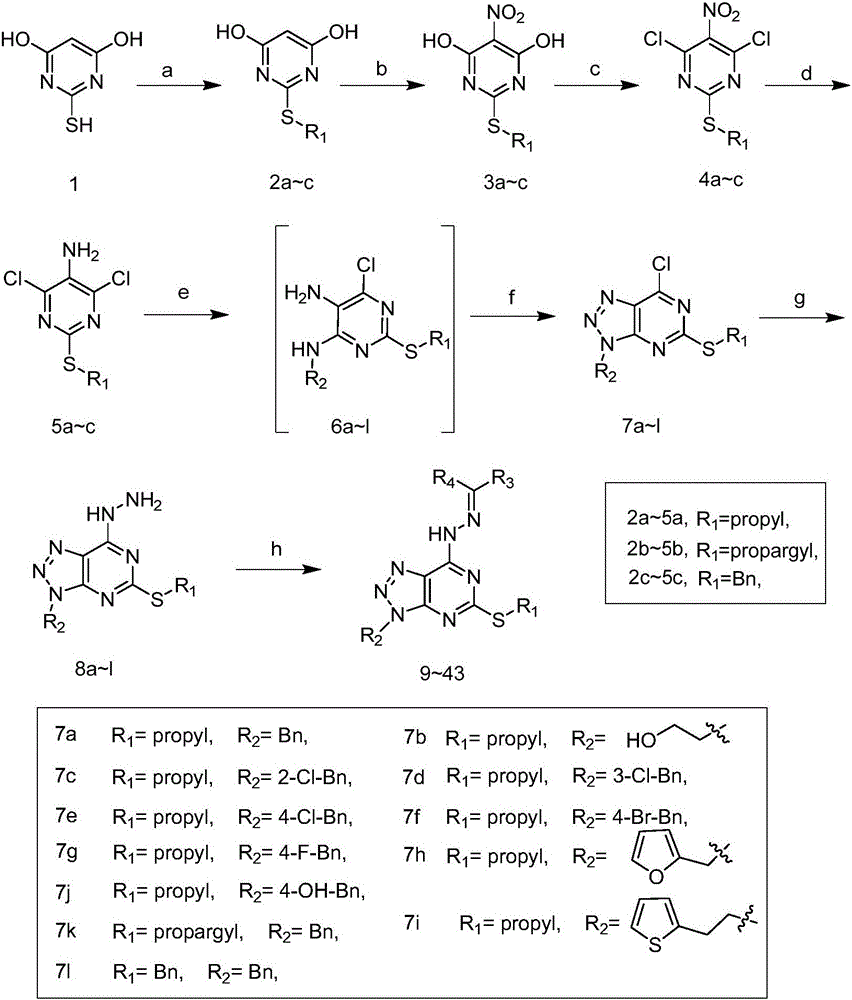
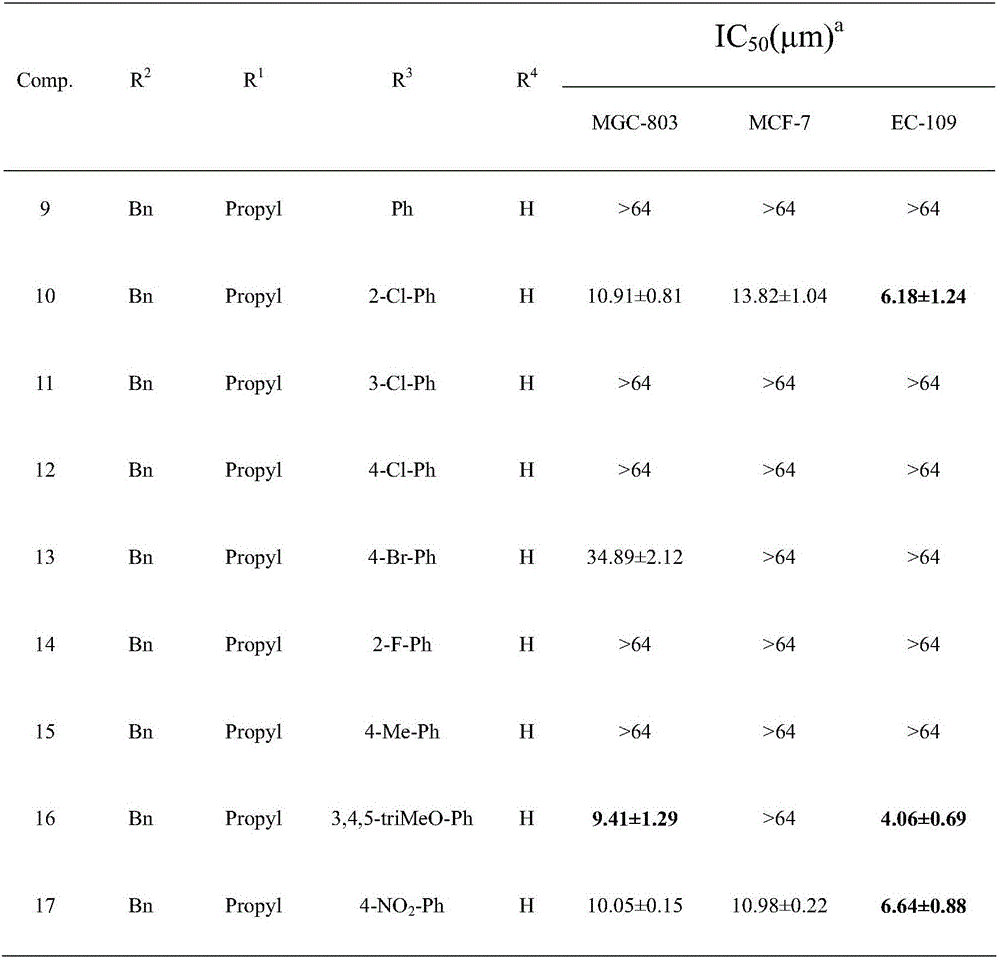
Pyrimidotriazole compounds containing hydrazone bonds as well as preparation method and application of pyrimidotriazole compounds
A triazole and compound technology, applied in the field of medicinal chemistry, can solve the problem of less research on anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 compound 2b, R 1 = Preparation of Propargyl
[0076] Add barbituric acid (3g, 1eq) and triethylamine (2.9ml, 1eq) to 30ml of methanol, heat to reflux, slowly add propyne bromide (1.8ml, 1eq) dropwise, and continue to reflux for 1 hour after the addition , cooled, and filtered with suction to obtain 3.7g of pink solid with a yield of 97%.
Embodiment 2
[0077] Example 2 Compound 3b, R 1 = Preparation of Propargyl
[0078] Under ice bath, carefully dissolve 3ml of fuming nitric acid in 6ml of acetic acid, then add 2.9g of compound 2b in batches, after the addition, continue to stir for 2 hours, then add the reaction solution to 18ml of ice water, filter with suction, Washed with water to obtain dark red powder 77.5%.
Embodiment 3
[0079] Example 3 Compound 4b, R 1 = Preparation of Propargyl
[0080] At room temperature, compound 3b (12.4g, 1eq) was dissolved in toluene, and added to 50ml of phosphorus oxychloride in batches, and DMA (12ml, 1.8eq) was slowly added dropwise, then heated to reflux, and reacted for 5 hours. Cool to room temperature, hydrolyze, then extract with EA, wash with water, then wash with saturated sodium carbonate solution, and dry the organic phase to obtain 13 g of brown crude compound 4 with a yield of 90.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com