Avian influenza virus trimer subunit vaccine and application thereof
A technology of influenza virus and trimer, which is applied in the field of medicine and can solve the problems of insufficient immunogenicity of monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Construction of recombinant expression plasmids for H7N9 HA1c-trimer and H7N9 HA1-monomer proteins
[0049] The following two HA1 recombinant expression fragments were designed:
[0050] a.H7N9 HA1c-trimer: 5′-SP+LAH+HA1+6×His+stop codon-3′;
[0051] b. H7N9 HA1-monomer: 5'-SP+HA1+6xHis+stop codon-3'.
[0052] in
[0053] SP is the GP67 signal peptide, and the amino acid sequence is SEQ ID NO: 14;
[0054] LAH is the trimer-forming region LAH (K403-N474) of influenza virus A / California / 07 / 2009 H1N1 HA protein, and its amino acid sequence is SEQ ID NO: 1;
[0055] HA1 is influenza virus A / Shanghai / 2 / 2013 H7N9 HA1 region D18-S340, and its amino acid sequence is SEQ ID NO: 2.
[0056] According to the codon preference of insect cells, the optimized coding nucleic acid sequence of H7N9 HA1c-trimer with SP and 6×His fragments removed is obtained as SEQ ID NO: 19, and then the full-length sequence is linked to the two restriction sites of EcoRI and XhoI. H7N9 ...
Embodiment 2
[0057] Example 2: Trial expression and identification of H7N9 HA1c-trimer and H7N9 HA1-monomer proteins
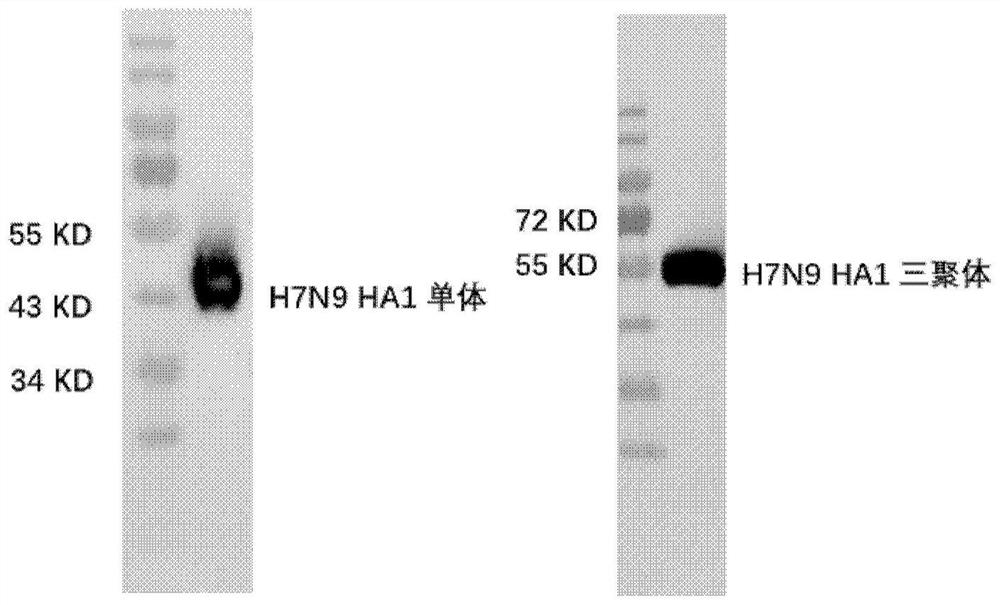
[0058] DH10Bac competent cells (purchased from Invitrogen) were transformed with the recombinant plasmid obtained in Example 1, cultured at 37°C overnight, and positive clones were identified by blue-white screening and PCR. Recombinant baculovirus DNA (Bacmid) was extracted and the correct recombinants were identified by sequencing. Insect cells Sf9 (Invitrogen) were transfected with Bacmid, and the culture supernatant was collected 3 days after transfection to obtain the first-generation recombinant baculovirus. After continuous expansion for 2 generations, the 3rd generation baculovirus was obtained. Viruses were collected and detected by Western blot (WB) using HRP-labeled Anti-His antibody (MBL).
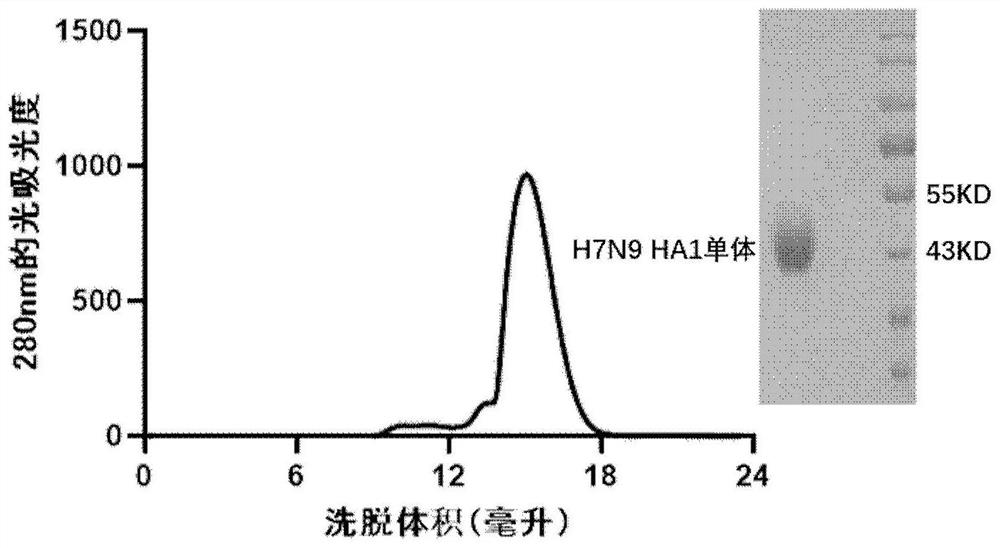
[0059] After WB detection, it was determined whether H7N9 HA1c-trimer and H7N9 HA1-monomer could be expressed normally, ( figure 1 ), the H7N9 HA1c-trimer construction w...
Embodiment 3
[0060] Example 3: Expression, purification and identification of H7N9 HA1c-trimer and H7N9 HA1-monomer proteins
[0061] After the fourth-generation amplification was performed with the third-generation baculovirus obtained in Example 2, the virus titer was determined. According to the titration results, a suitable virus MOI was selected to infect Sf9 or the insect cell line Hi5 (Invitrogen) for expression, and the cell supernatant was collected by centrifugation 48 hours later.
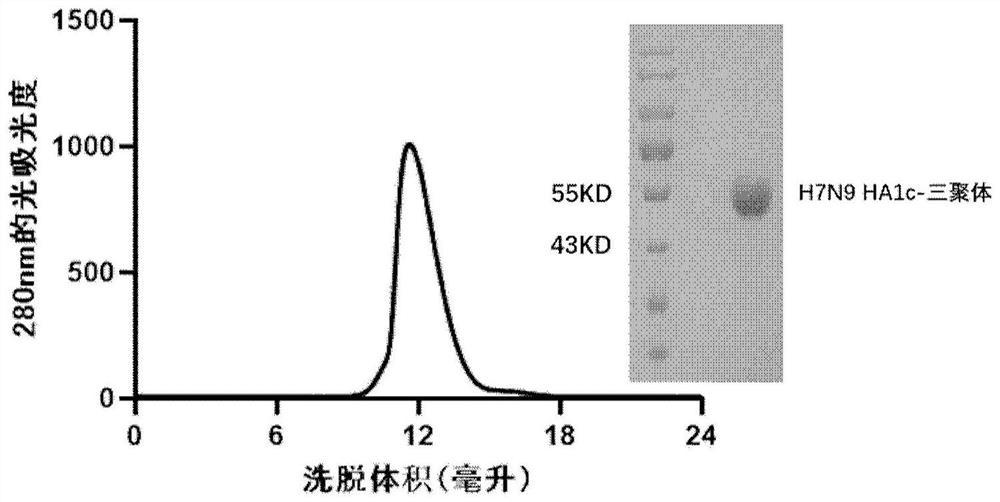
[0062] The collected supernatant was centrifuged at 5000 rpm for 30 min, filtered through a 0.22 μm filter, bound to a HisTrap excel column (5 mL, GE Healthcare), and non-specifically bound was eluted with 20 mM Tris, 150 mM NaCl, pH 8.0, 20 mM imidazole. protein, the target protein was eluted with 20 mM Tris, 150 mM NaCl, pH 8.0, 100 mM imidazole. The target protein was collected and concentrated and then subjected to molecular sieve chromatography Superdex 200 Increase 10 / 300GL or Superdex 200 Hil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com