Recombinant haemophilus parasuis immunoprotecive antigen HbpA2 and preparation method thereof
A technology for recombining Escherichia coli and recombinant bacteria, applied in the field of genetic engineering, can solve the problems of poor protection of Haemophilus parasuis and only 50% immune protection effect, and achieve the prevention of Haemophilus parasuis disease and clinical application prospects Good, high antibody level results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Preparation of Haemophilus parasuis immunoprotective antigen HbpA2 of the present invention
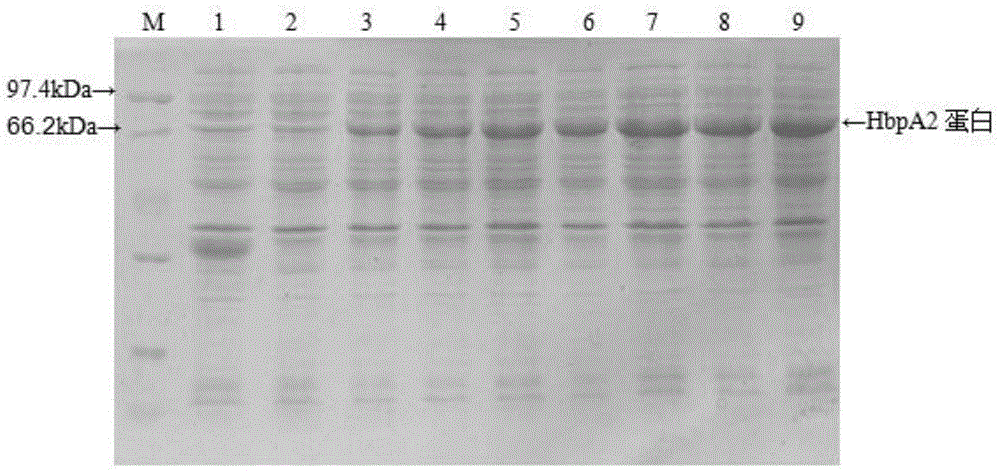
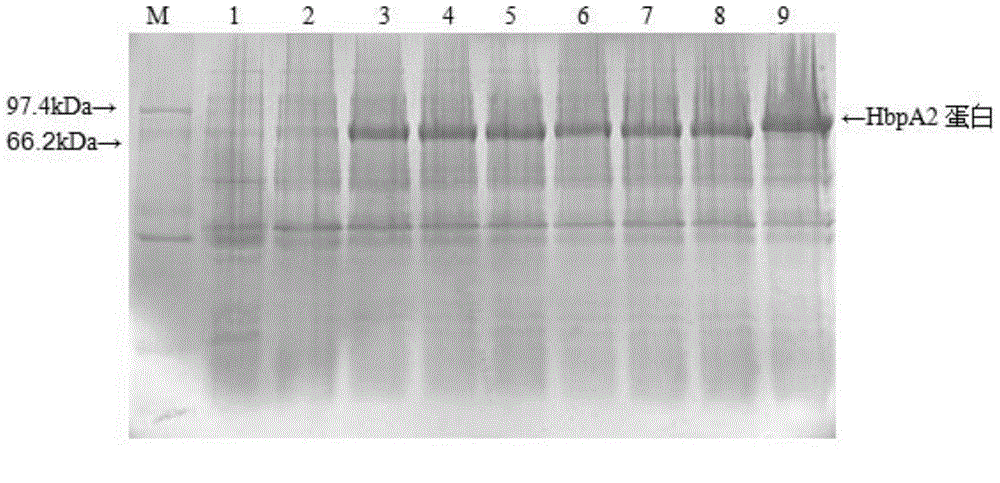
[0036] 1. Recombinant expression
[0037] 1.1 Cloning of HbpA2 gene and construction of expression vector
[0038] 1.1.1 Primer design and synthesis
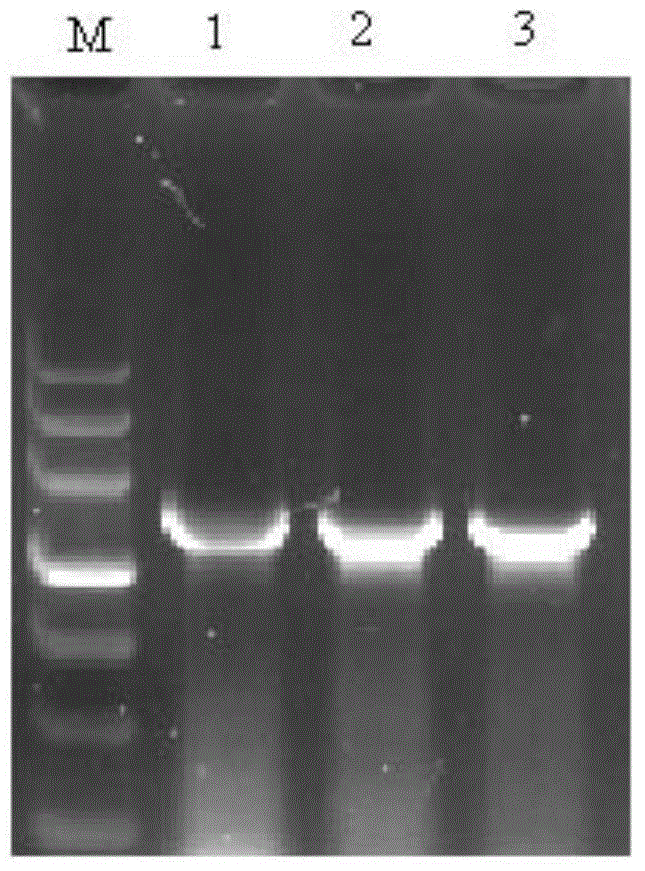
[0039]According to the HbpA2 gene sequence of Haemophilus parasuis (Gene ID: 7278927), a pair of primers were designed with biological software Premier Primer5.0 to amplify the HbpA2 gene.
[0040] Upstream primer: 5'-AGTACTCCGACAAATACATTGGTCAACTGT-3';
[0041] Downstream primer: 5'-CTCGAGTTAAGGCTTCAGACTTACGCCATA-3';
[0042] 1.1.2 PCR amplification of HbpA2 gene
[0043] Using the genomic DNA of Haemophilus parasuis CVCC3361 as a template, PCR amplification was carried out according to the conventional method, the amplification system was 25 μL, and 4 replicates were performed. Amplification system:
[0044] Material
volume
genome template
1μL
Primer (upstream, downstream)
2...
experiment example 1
[0131] Experimental example 1 In vivo protective effect of recombinant protein HbpA2 of the present invention
[0132] 1. Experimental method
[0133] 1.1 Determination of the median lethal dose (LD50) of Haemophilus parasuis CVCC3361
[0134]1.1.1 Determination of LD0 and LD100
[0135] 10 mice were used as a group, and each mouse was intraperitoneally injected with live bacterial solution 1×10 9 CFU (LD0 estimated in the pre-experiment), count the death of the mice after 7 days, if two or more died, the challenge dose was reduced by 0.1×10 9 CFU, if all survive, the attack dose will increase by 0.1×10 9 CFU until only one mouse in a group dies, then the adjacent low dose is LD0. Obtain LD100 by the same method.
[0136] 1.1.2 Determination of the challenge dose of the experimental group
[0137] The mice were randomly and equally divided into 7 groups, with 10 mice in each group, of which 7 groups were control groups, and groups 1-6 were test groups. Calculate the r v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com