Methods and kits for the detection of an infection in subjects with low specific antibody levels
A specific, subject-based technique, applied to antibodies against and/or breakthrough infection, detection against latent infection, chronic infection, re-infection, capable of addressing below or just at detection levels, hindering detection specificity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
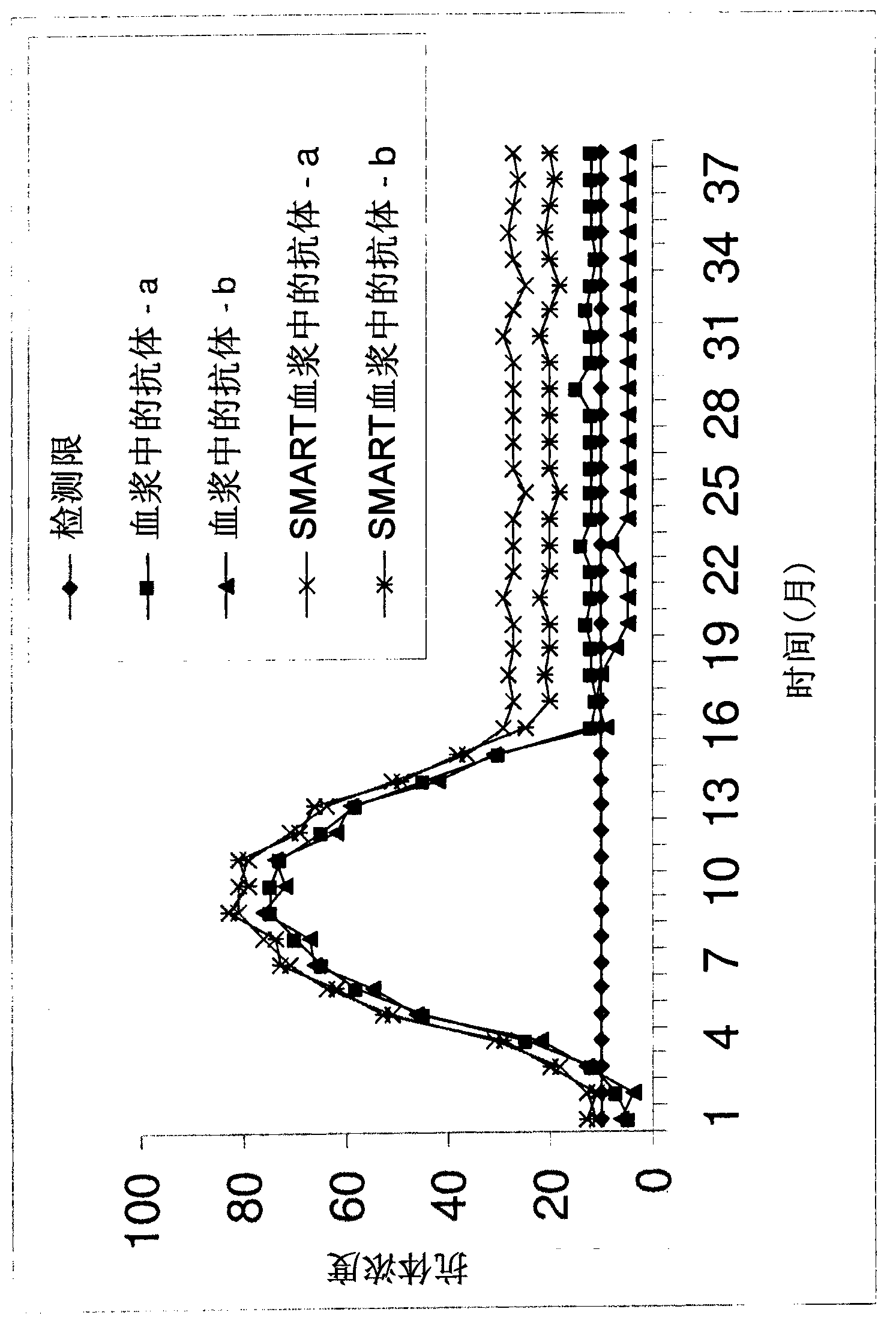
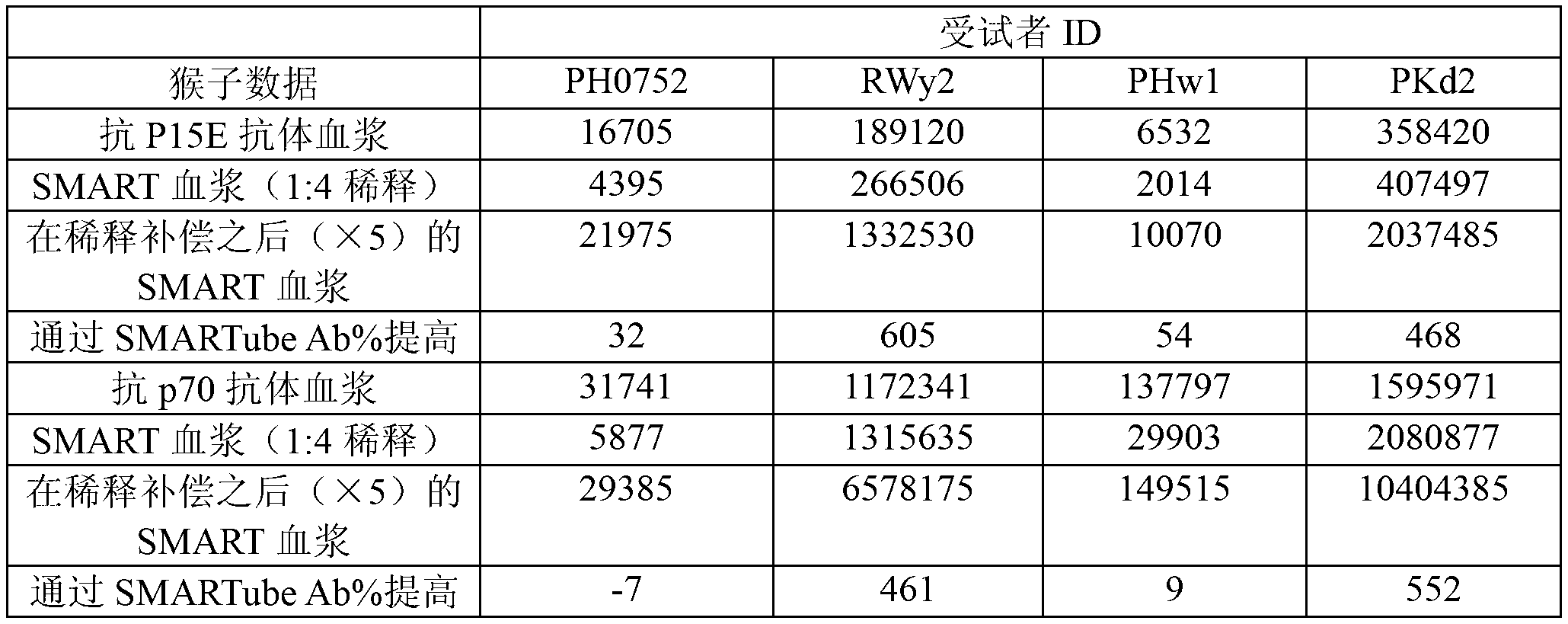
[0169] Example 1: Activators increase seroresponse to xenotropic murine leukemia virus (XMRV) infection Specific antibodies in subjects who should be positive
[0170] Blood from subjects known to be infected with XMRV was collected in anticoagulants, brought to the laboratory, and within 24 hours at room temperature, 1 ml of blood was transferred to tubes containing mitogen. Test the rest of the plasma for XMRV antibodies ( figure 1 , solid line) and remain for any additional future comparison tests. Incubate blood for 3-5 days in tubes containing mitogens (5% CO 2 , 37°C), the treated plasma was then collected and tested for XMRV antibodies using the same kit / assay used to test untreated plasma.
[0171] XMRV antibody concentrations were higher in mitogen-treated plasma than in untreated plasma (Table 1). Therefore, XMRV infection of processed plasma can be diagnosed with higher sensitivity.
[0172] Table 1. In untreated and mitogen-treated blood from XMRV-infecte...
Embodiment 2
[0179] Example 2: Anti-IGD further increases xenotropic murine leukemia virus (XMRV) infection Raised specific antibodies to the activator in seropositive subjects
[0180] Blood samples collected from individuals infected with XMRV whose plasma antibody levels have fallen to at or below assay detection levels (eg, OD readings near the cutoff value). Aliquots of 1 ml of fresh, non-clotted whole blood (or PBMC) were incubated in anti-IgD, anti-IgD and mitogen, or mitogen alone.
[0181] Table 3: After three days of incubation with anti-IgD, mitogen, or anti-IgD and mitogen After that, the OD reading of the anti-XMRV antibody:
[0182] sample#
plasma (unprocessed)
anti-IgD
Anti-IgD and mitogens
mitogen
1
0.110
0.120
0.400
0.300
2
0.200
0.350
0.600
0.550
3
0.101
0.150
0.380
0.200
4
0.090
0.110
0.180
0.140
5
0.087
0.115
0.240
0.170
...
Embodiment 3
[0185] Example 3: Activators Improve Seropositive Hepatitis E Virus (HEV) Infection Specific antibodies in the subject
[0186] Blood collected in anticoagulant from the local population in Beijing was brought to the laboratory (within 24 h at RT) and 1 ml of blood was transferred to a tube containing activator (SMARTube TM , which can be obtained from http: / / www.smartube-bio.com / ?CategoryID=190 , accessed September 6, 2011) in the container. The remainder of the plasma (untreated) was tested for HEV antibodies and kept for any additional future comparative testing. Incubate the blood in the container for 3-5 days (5% CO 2 , 37°C), plasma was then collected and tested for HEV antibodies using the same kit / assay used to test unprocessed blood samples.
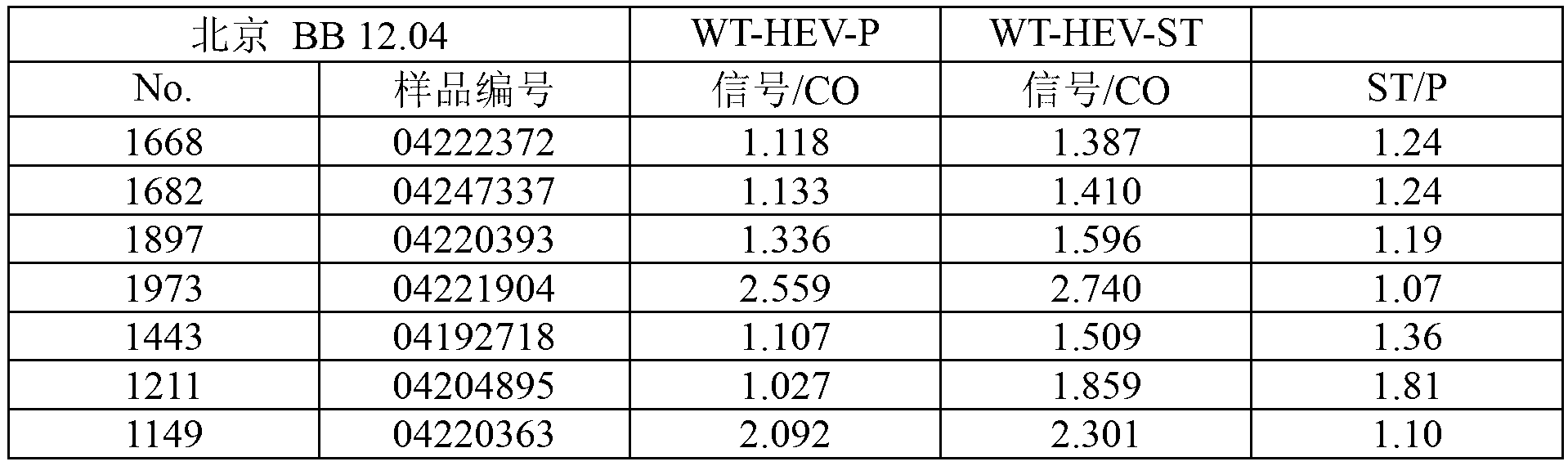
[0187] Table 4: HEV antibody levels after stimulation of whole blood with activators (ELISA)
[0188]
[0189] Table 4 shows the results for 7 / 15 seropositive samples in which antibody levels increased after stimul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com