New coronavirus mRNA vaccine for targeted stimulation of humoral immunity and cellular immunity
A vaccine and virus technology, applied in the field of immunity, can solve problems such as immune escape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
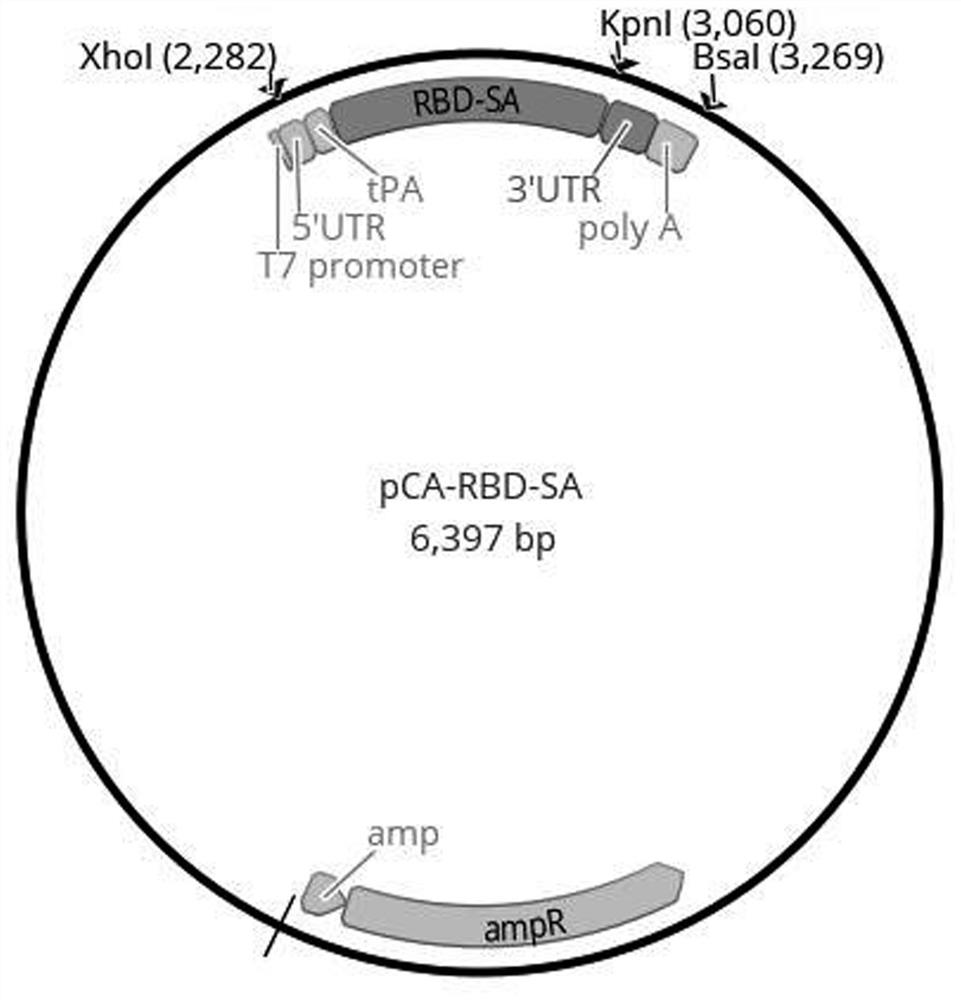
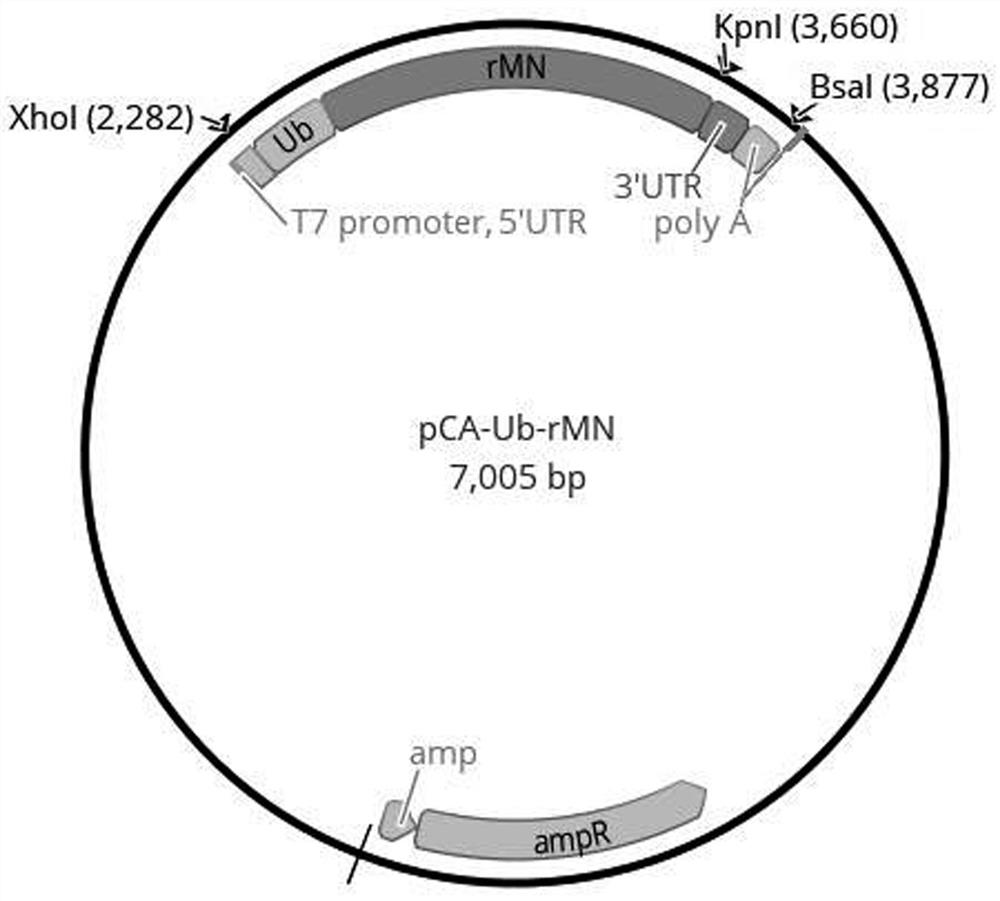
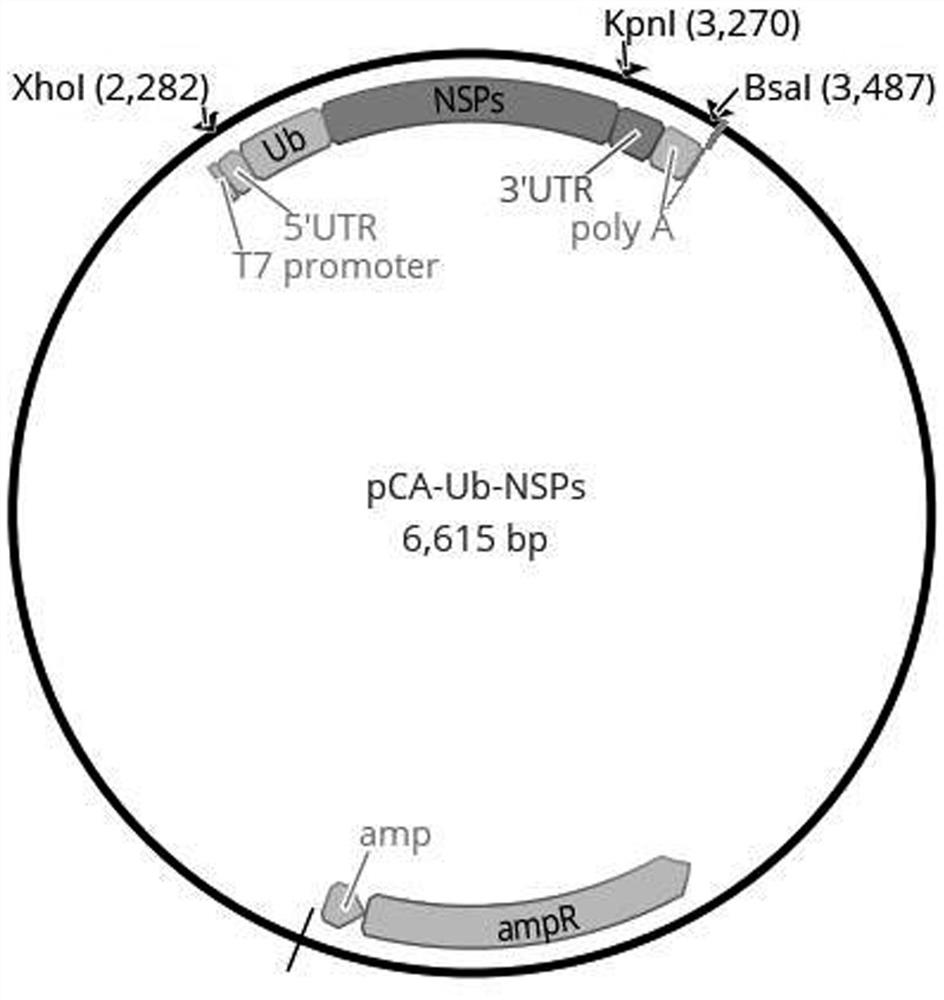
[0068] This embodiment provides a combination of antigen expression vectors for a novel coronavirus mRNA vaccine. The combination of antigen expression vectors includes three antigen expression vectors, pCA-RBD-SA, pCA-Ub-rMN and pCA-Ub-NSPs, respectively.
[0069] These antigen expression vectors refer to Figures 1~3 , wherein, the pCA-RBD-SA plasmid has an antigen coding region, and the antigen coding region includes the sequence encoding the tPA signal peptide and the sequence encoding the RBD-SA fragment connected in sequence, and the sequence encoding the RBD-SA fragment 3' A sequence encoding a Flag tag and a stop codon were also inserted at the ends (Flag sequence and stop codon are not shown in the figure). The upstream and downstream of the antigen coding region are also connected with T7 promoter, 5'-end untranslated region (5'-UTR), 3'-end untranslated region (3'-UTR), and polyadenylation (polyA).
[0070] The pCA-Ub-rMN plasmid has an antigen coding region, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com