A new coronavirus mRNA vaccine targeting humoral and cellular immunity
A vaccine and virus technology, applied in the field of immunity, can solve problems such as immune escape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
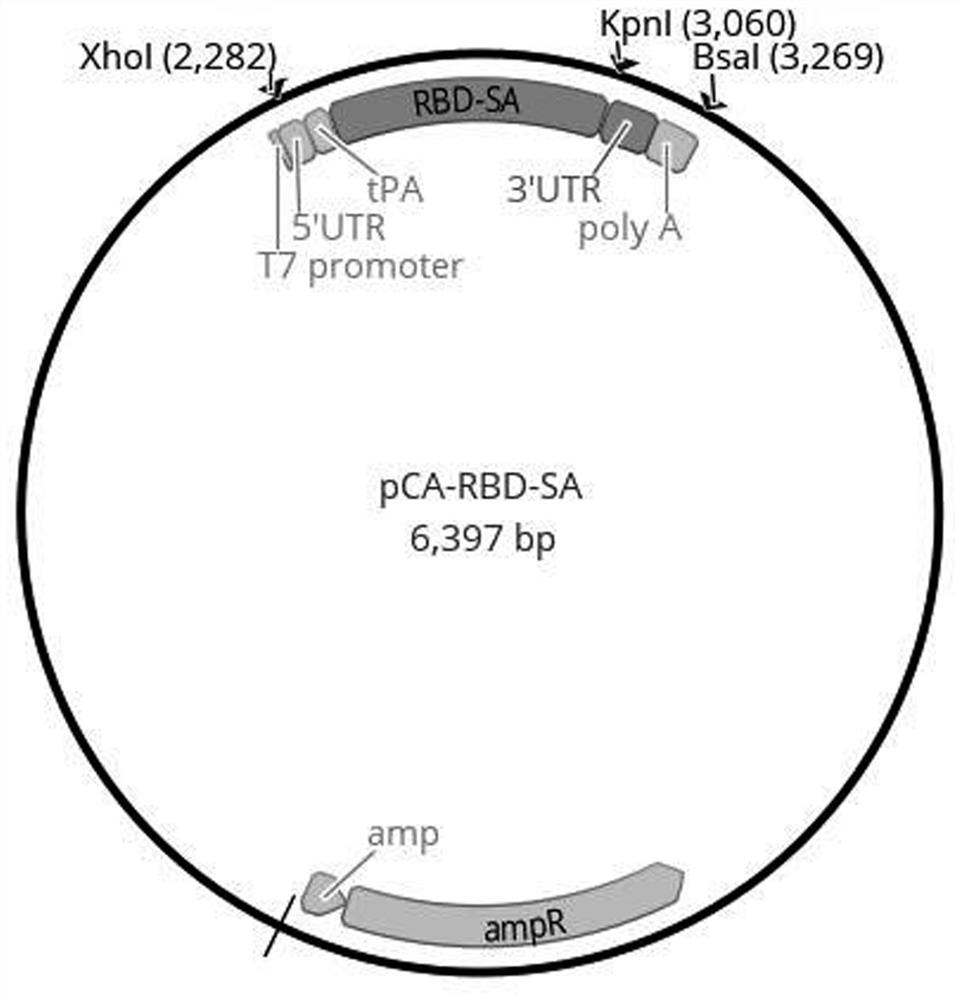
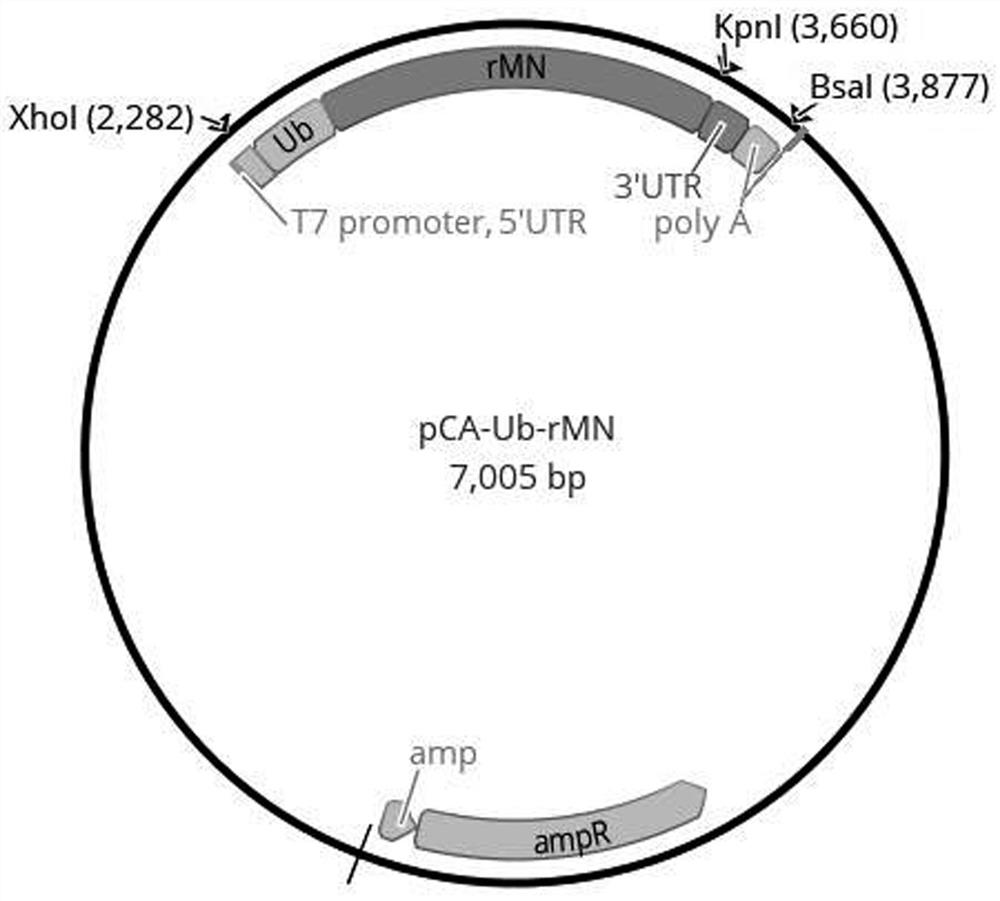
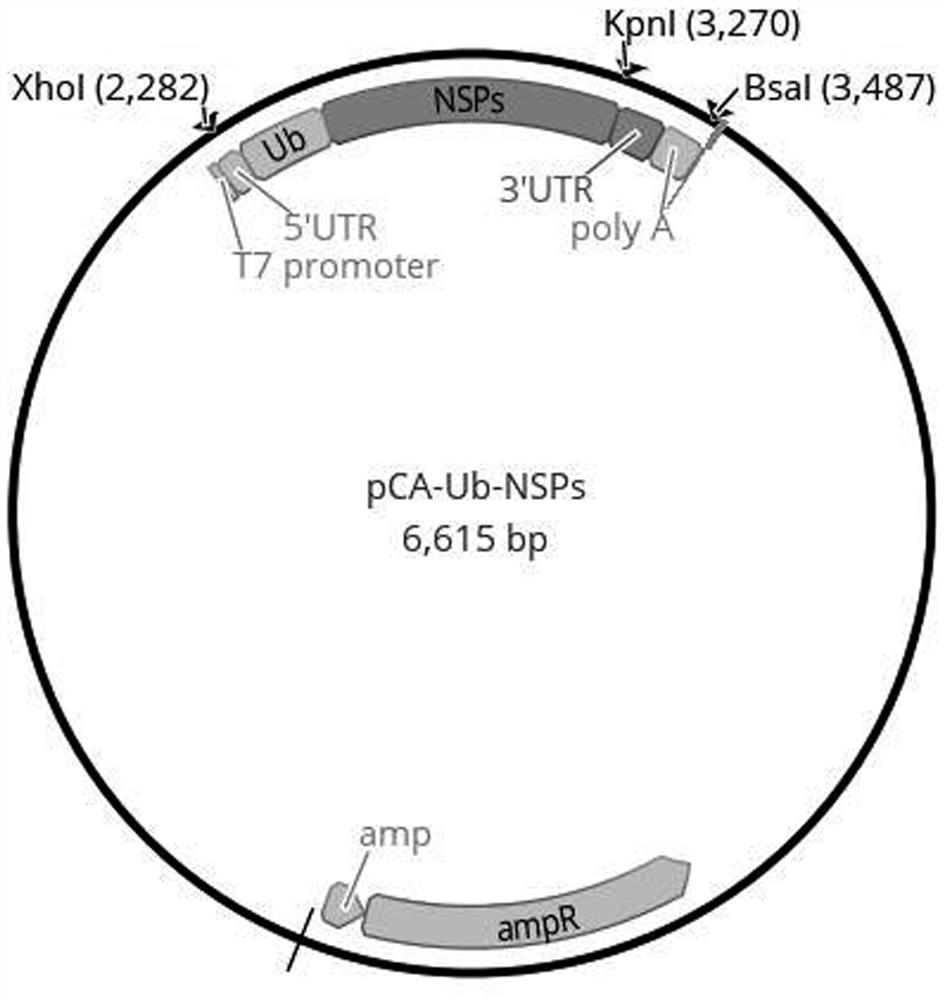
[0068] This embodiment provides a combination of antigen expression vectors for the novel coronavirus mRNA vaccine. The combination of antigen expression vectors includes three antigen expression vectors, namely pCA-RBD-SA, pCA-Ub-rMN and pCA-Ub-NSPs.
[0069] These antigen expression vectors are respectively referred to Figure 1~3 , wherein, there is an antigen coding region on the pCA-RBD-SA plasmid, and the antigen coding region includes the sequence of coding tPA signal peptide and the sequence of coding RBD-SA fragment connected in sequence, at the 3′ of the sequence of coding RBD-SA fragment A sequence encoding a Flag tag and a stop codon were also inserted at the end (the Flag sequence and stop codon are not shown in the figure). The T7 promoter, 5' untranslated region (5'-UTR), 3' untranslated region (3'-UTR), and polyadenylic acid (polyA) are also connected upstream and downstream of the antigen coding region, respectively.
[0070] There is an antigen coding regio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com