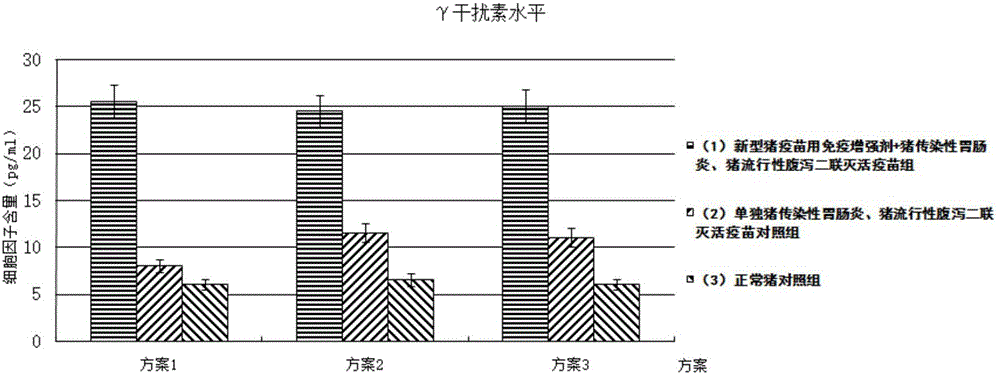
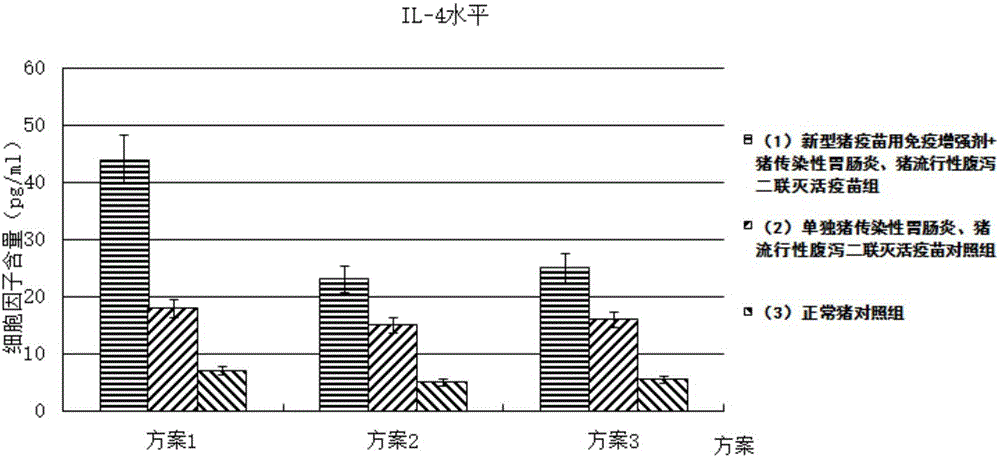
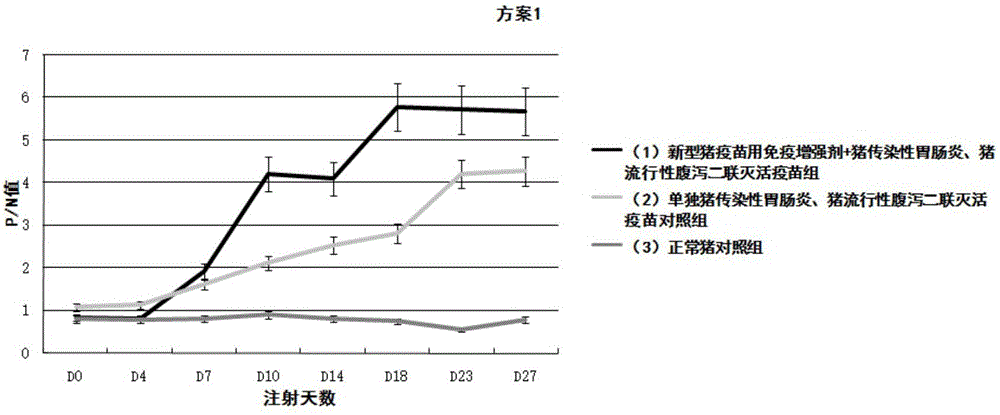
Immune enhancer for pig vaccine, and preparation method and application thereof
An immune enhancer and vaccine technology, applied in the field of veterinary biological products, can solve the problems of no effective vaccine prevention and control of infectious diseases, reduced vaccine protection, low vaccine immune efficacy, etc., to improve the level of cellular immune response, easy to store, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of recombinant porcine interferon α, recombinant porcine interferon γ, and recombinant porcine IL-2
[0037] The preparation of recombinant porcine interferon α, recombinant porcine interferon γ, and recombinant porcine IL-2 comprises the following steps:
[0038] 1) Preparation of recombinant porcine interferon α
[0039] Construction of engineering Escherichia coli containing recombinant porcine interferon α target gene → engineering bacteria fermentation → bacterial liquid crushing → centrifugation to obtain the supernatant containing the target protein → protein purification → intermediate product inspection; for the preparation method, please refer to the published on October 22, 2008 Chinese patent CN101289666.
[0040] 2) Preparation of recombinant porcine interferon gamma
[0041] Construction of engineering Escherichia coli containing recombinant porcine interferon gamma target gene → engineering bacteria fermentation → bacterial liquid crushing →...
Embodiment 2
[0067] Immunopotentiator for preparing porcine vaccine
[0068] The new immunopotentiator for porcine vaccines of this embodiment includes porcine genetically engineered cytokine complexes and lyoprotectants.
[0069] The porcine genetically engineered cytokine complex comprises the following components in parts by weight: 50 parts by weight of recombinant porcine interferon α, 25 parts by weight of recombinant porcine interferon γ, 25 parts by weight of recombinant porcine interferon α, and 25 parts by weight of recombinant porcine interferon α The nucleotide sequence of the coding gene is shown in SEQ ID No.1, the nucleotide sequence of the coding gene of the recombinant porcine interferon gamma is shown in SEQ ID No.2, and the coding of the recombinant pig IL-2 The nucleotide sequence of the gene is shown in SEQ ID No.3.
[0070] The lyoprotectant includes skimmed milk powder, glucose and serum albumin, and the concentration of each component in the immune enhancer is as f...
Embodiment 3
[0078] Immunopotentiator for preparing porcine vaccine
[0079] The new immunopotentiator for porcine vaccines of this embodiment includes porcine genetically engineered cytokine complexes and lyoprotectants.
[0080] The porcine genetically engineered cytokine complex comprises the following components in parts by weight: 60 parts by weight of recombinant porcine interferon α, 20 parts by weight of recombinant porcine interferon γ, 20 parts by weight of recombinant porcine IL-2, the recombinant porcine interferon α The nucleotide sequence of the coding gene is shown in SEQ ID No.1, the nucleotide sequence of the coding gene of the recombinant porcine interferon gamma is shown in SEQ ID No.2, and the coding of the recombinant pig IL-2 The nucleotide sequence of the gene is shown in SEQ ID No.3.
[0081] The lyoprotectant includes skimmed milk powder, glucose and serum albumin, and the concentration of each component in the immune enhancer is as follows: skimmed milk powder 5g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com