Monoclonal antibody for identifying PCV2 virus-like particles and application thereof in qualitative and quantitative detection of PCV2 virus-like particles
A monoclonal antibody and polyclonal antibody technology, applied in the field of biomedicine, can solve the problems of inability to specifically identify the structural integrity and purity of VLPs, inability to distinguish non-specific proteins and broken subunits, and expensive VLP particle structure detection instruments. , to achieve the effect of fast and accurate protein content, good linear relationship and good precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of monoclonal antibody specifically recognizing porcine circovirus type 2 virus-like particles
[0052] 1. Preparation of PCV2 VLPs
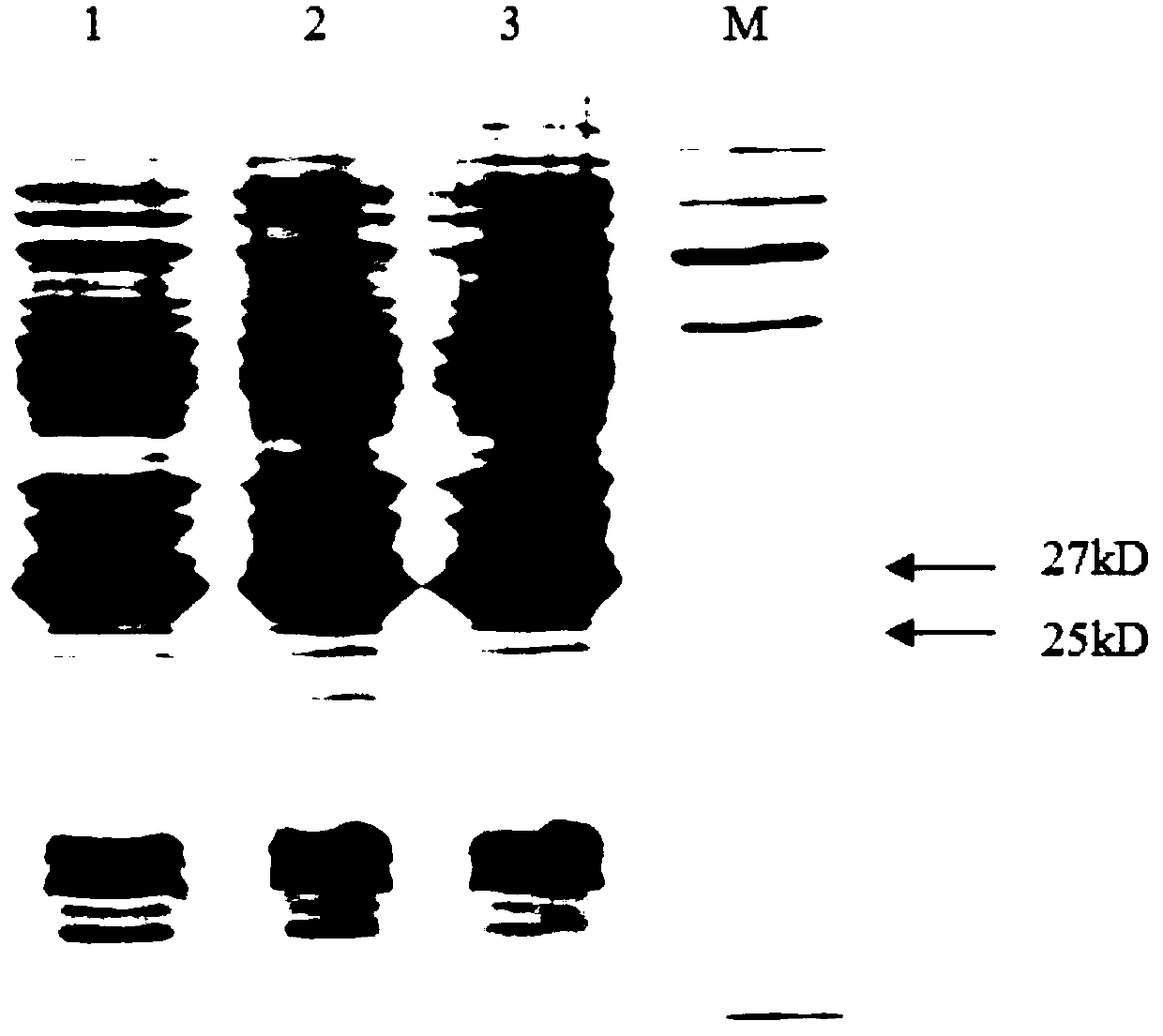
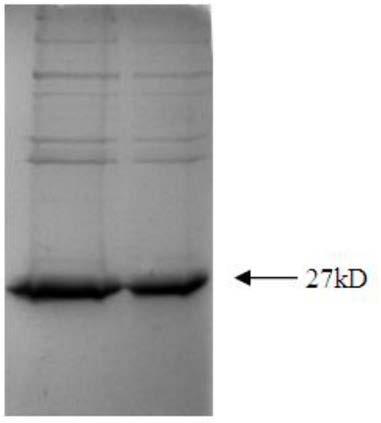
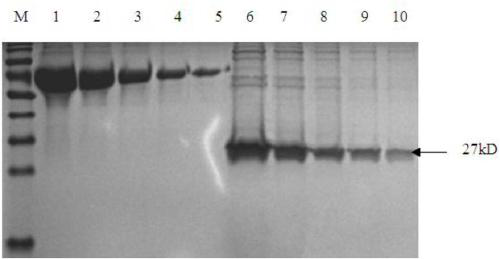
[0053] (1) Clone the PCV2 cap protein gene and connect it to the PET-28a expression vector. After the correct insertion of the clone was confirmed by restriction enzyme digestion, the sequence of the recombinant plasmid was confirmed to be correct by sequencing. The recombinant plasmid was transformed into the expression strain BL21 to induce expression, and the collected cells were broken by a high-pressure homogenizer, redissolved after ammonium sulfate precipitation, and the results of SDS-PAGE electrophoresis showed that there was an obvious band at the size of 27KD, which was consistent with the molecular weight of the cap protein ( figure 1 ).
[0054] (2) The expressed protein was identified by western blot, the bacterial protein was transferred to PEDV membrane by SDS-PAGE, and identified by his tag antibody...
Embodiment 2
[0064] Example 2 Preparation of Double Antibody Sandwich ELISA Quantitative Kit
[0065] 1. Using 3H9 monoclonal antibody as coating antibody, rabbit anti-PCV2 VLPs polyclonal antibody and HRP-labeled goat anti-rabbit polyclonal antibody as detection antibody, a double sandwich ELISA detection method was established to analyze the protein concentration of VLPs.
[0066] 2. The operation process of the double-antibody sandwich ELISA quantitative kit
[0067] (1) Dilute the 3H9 monoclonal antibody with coating solution (50mM carbonate coating buffer, pH9.6) at 1:12800, and add 100 microliters per well to the ELISA plate. Coating overnight at 4°C;
[0068] (2) Wash the coated ELISA plate 4 times with PBST solution (0.1mol / L PBS solution containing 0.5v / v% Tween20, pH 7.6, diluted 10 times when used, the same below), 200 microliters per well , 5 minutes each time. Add 200 microliters of blocking solution (0.01M PBS solution containing 1w / v% BSA, pH 7.6) to the ELISA plate, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com