Expressed rabies virus glycoprotein optimized through gene modification and monoclonal antibody and application thereof
A rabies virus and monoclonal antibody technology, applied in the field of immunology and biology, to achieve the effect of increasing immunogenicity and facilitating screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Obtaining of Modified G Protein Gene G3cDNA
[0040] 1. Use bioinformatics software to analyze the sequence of the glycoprotein extramembrane region of rabies virus HEP-Flury strain, and select the 100-307 amino acids in the dominant segment of the antigenic epitope (the antigenic epitope analysis of RV GP is shown in the attached figure 1 -A), modify the amino acid codon according to the codon preference of Escherichia coli, the sequence is shown in the attached figure 1 As shown in -B, BamHI and HindIII restriction sites were introduced at the 5' end and 3' end, respectively, with a theoretical length of 648 bp. The sequence was synthesized by Shanghai Yingjun Biotechnology Co., Ltd. and named G3.
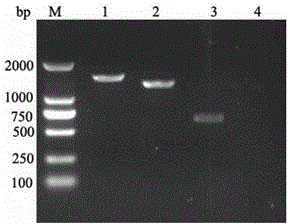
[0041] 2, figure 2 As a result of gel electrophoresis of the G3PCR product, the amplified target gene fragment G3 double digestion purified product (swimming lane 3) is consistent with the expectation, which is 648bp (including double restriction sites and prot...
Embodiment 2
[0042] Example 2 Construction of pET32-G3 recombinant plasmid
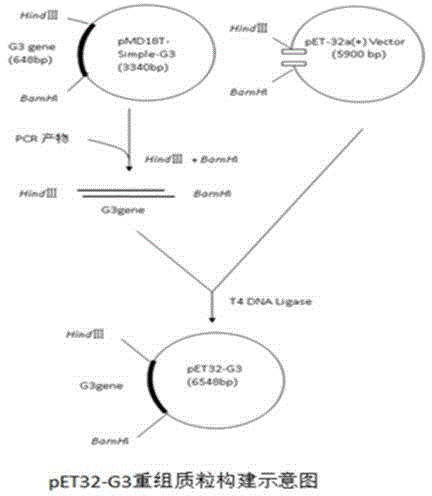
[0043] 1. The PCR amplification product of the G3 gene was digested by BamH I and Hind III, and after recovery, it was inserted into the BamHI and Hind III double digestion window of the pET-32a(+) vector to obtain the recombinant plasmid of the G3 fusion protein gene fused with the His tag , build process like image 3 shown; image 3 The amplified product of the G3 gene was double-digested with BamH I and Hind III, and inserted into the BamH I and Hind III double-digestion window of pET-32a(+), to obtain a recombinant plasmid containing the XXX-G3 fusion protein gene.
[0044] 2. Transform Escherichia coli BL21 with the recombinant plasmid, spread the Escherichia coli BL21 on the LB plate containing ampicillin, overnight at 37°C, pick the plasmid transformed bacteria for amplification the next day, extract the plasmid DNA for enzyme digestion identification, and On 1.0% agarose gel electrophoresis, it can be s...
Embodiment 3
[0046] Example 3 Induced expression of G3 fusion protein fused with His tag
[0047] 1. Pick a single colony identified to contain the recombinant expression plasmid pET32-G3 in the Amp-containing + (100 μg / mL) in LB liquid medium, culture overnight at 37°C. Take the above-mentioned culture bacteria and inoculate them into fresh Amp-containing + (100μg / mL) 2×YT culture solution, cultivated at 37°C until the OD600 value was 0.5, then added IPTG to a final concentration of 1.0mmol / L to induce the expression of GP, and continued to cultivate at 37°C for 4h, and the induced product was 12000r After centrifuging for 1 min at 100 Å / min, collect the supernatant and cells respectively, add the same volume of 2×SDS-PAGE Loading Buffer to the supernatant, add 200 μL 1×SDS-PAGE Loading Buffer to the cells, freeze and thaw three times, boil for 10 min, Centrifuge at 12000r / min for 10min to remove large protein fragments, and take the supernatant for SDS-PAGE protein electrophoresis imme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com