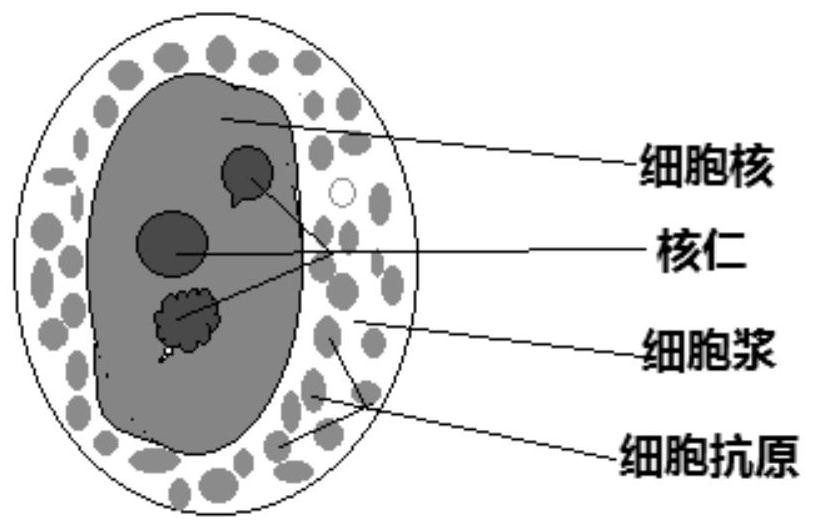
Human cell whole form immunofluorescence staining method and kit
An immunofluorescence staining and human cell technology, applied in the field of human cell full-form immunofluorescence staining methods and kits, can solve the problem of inability to distinguish nucleolus and nucleoplasm, low sensitivity of nucleolar color development, and inability to clearly visualize nucleoli. shape and structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Cell characteristic direct immunofluorescence cell staining process
[0085] Main reagents:
[0086] ① Fluorescent dye (Alexa 647) labeled nucleolin (Nucleolin), orange red;
[0087] ② Fluorescent dye (Alexa 594) Marked nucleolar fibrin (fibrilarin), red;
[0088] ③Nuclear fluorescent dye: DAPI, blue-purple;
[0089] ④ fluorescent dye (Alexa 488) labeled anti-CK7, CK18, CK19 monoclonal antibody, green;
[0090] Pay attention to the wavelength and color matching of each fluorescent dye.
[0091] Cell fixatives: methanol, acetone, paraformaldehyde.
[0092] The cell blocking solution includes: pH 7.2-7.4 PBS (containing 0.5-10.0% BSA or calf serum).
[0093] The washing solution includes: pH7.2-7.4PBS (containing Tween-20).
[0094] The diluent includes: pH 7.2-7.4 PBS (containing 0.1-1.5% BSA or calf serum), adding appropriate concentration of fluorescent labeled antibody or non-labeled antibody to form antibody binding reaction solution.
[0095]...
Embodiment 2
[0107] Example 2: Cell characteristic indirect immunofluorescence cell staining process
[0108] Main reagents:
[0109] ① Nucleolin antibody.
[0110] ② nucleolar fibrin (fibrillarin) antibody.
[0111] ③Nuclear fluorescent dye: DAPI, blue-purple.
[0112] ④ fluorescent dye (Alexa 488) labeled anti-CK7, CK18, CK19 monoclonal antibody, green.
[0113] ⑤Apoptosis susceptibility protein antibody—goat anti-rabbit (Alexa 488) Fluorescently labeled antibodies.
[0114] ⑥ Goat anti-rabbit IgG (Alexa 594) labeled fluorescent secondary antibody.
[0115] Pay attention to the wavelength and color matching of each fluorescent dye.
[0116] Cell fixatives: methanol, acetone, paraformaldehyde.
[0117] The cell blocking solution includes: pH 7.2-7.4 PBS (containing 0.5-10.0% BSA or calf serum).
[0118] The washing solution includes: pH7.2-7.4PBS (containing Tween-20).
[0119] The diluent includes: pH 7.2-7.4 PBS (containing 0.1-1.5% BSA or calf serum), adding appropriate c...
Embodiment 3
[0133] Example 3: Human blood cell staining
[0134] Main reagents:
[0135] ① Fluorescent dye (Alexa 647) labeled nucleolin (Nucleolin), orange red.
[0136] ② Fluorescent dye (Alexa 594) labeled fibrillin, red.
[0137] ③Nuclear fluorescent dye: DAPI, blue-purple.
[0138] ④ fluorescent dye (Alexa 488) Monoclonal antibody labeled CD45, (leukocytes) in green.
[0139] Pay attention to the wavelength and color matching of each fluorescent dye.
[0140] Cell fixatives: methanol, acetone, paraformaldehyde.
[0141] The cell blocking solution includes: pH 7.2-7.4 PBS (containing 0.5-10.0% BSA or calf serum).
[0142] The washing solution includes: pH7.2-7.4PBS (containing Tween-20).
[0143] The diluent includes: pH 7.2-7.4 PBS (containing 0.1-1.5% BSA or calf serum), adding appropriate concentration of fluorescent labeled antibody or non-labeled antibody to form antibody binding reaction solution.
[0144] Cell membrane and nuclear membrane permeabilization solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com