Organic recycling with metal addition
a technology of organic recycling and metal addition, which is applied in the direction of nitrogenous fertilisers, animal corpse fertilisers, applications, etc., can solve the problems of difficult to obtain accurate flow rate control, low analysis of fertilizers with regard to plant nutrient value, and significant world-wide problem of sewage sludge discharge, etc., to achieve the effect of preventing fluid flow, and reducing the viscosity of slurry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
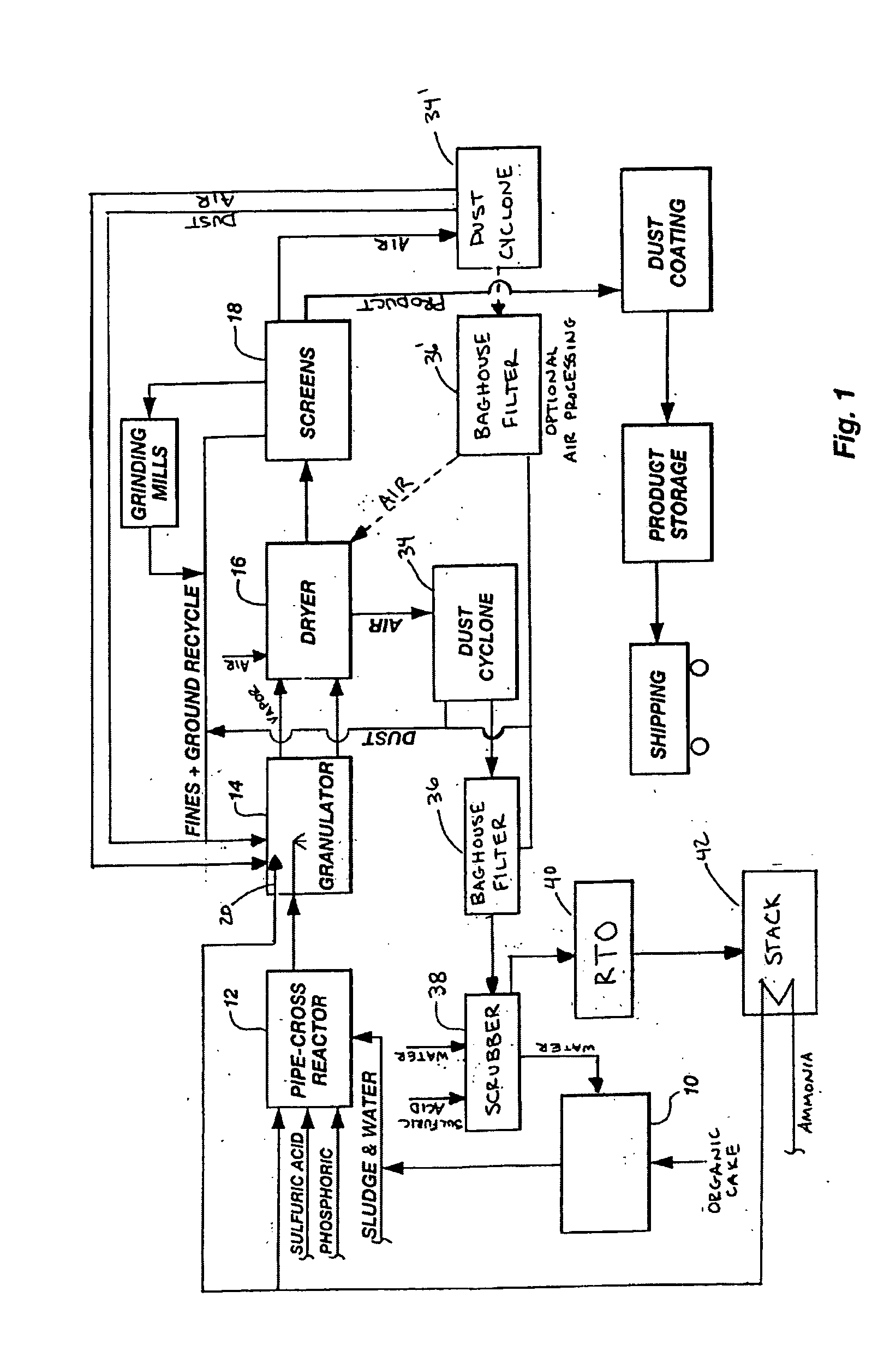
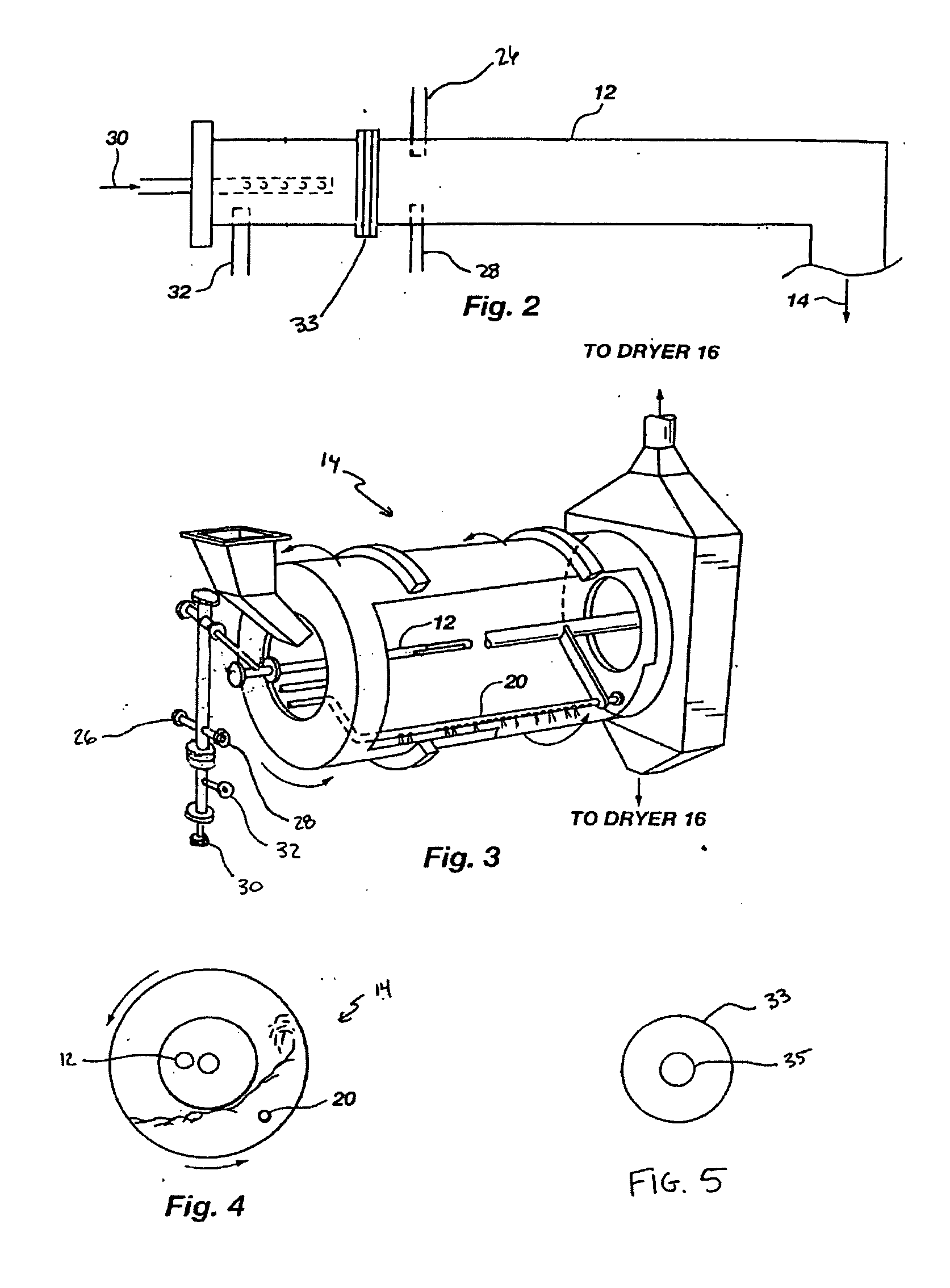
In an agitation tank, 6700 kilograms / hour (7.4 tons / hour) of sewage sludge were mixed with 37 liters per minute (ten gallons / minute (gpm)) of scrubber water to form a slurry. The slurry was of such a consistency (a solids content varying between 10% and 27%) that it can be pumped with a positive displacement pump or other suitable pump to a pipe-cross reactor equipped to receive ammonia, sulfuric acid, phosphoric acid, sewage sludge, and water. The pipe-cross reactor had a diameter of approximately four inches and was forty feet long. The pipe-cross reactor terminated in a rotating drum granulator. The rotating drum granulator was six feet in diameter and twenty feet long.
The slurry was added to the pipe-cross reactor and reacted with 8.6 gpm 99.5% ammonia, 8.6 gpm sulfuric acid (93%), and 2.6 gpm phosphoric acid (54% P2O5). The temperature of the pipe-cross reactor (due to the exothermic reaction between the acid and the base) was maintained at about 149° C. (300° F.) with moist...
example 2
The process of Example 1 is repeated in a tubular reactor rather than a pipe cross reactor. In an agitation tank, 6700 kilograms / hour (7.4 tons / hour) of sewage sludge are mixed with 37 liters per minute (ten gallons / minute (gpm)) of scrubber water to form a slurry. The slurry is of such a consistency that it can be pumped with a positive displacement pump or other suitable pump to a tubular reactor equipped to receive ammonia, sulfuric acid, phosphoric acid, sewage sludge, and water. The tubular reactor preferably has a diameter of approximately 1.5 to 30 cm and a length of 2 to 10 meters, preferably 5 to 8 meters. The reactor terminates in a rotating drum granulator. The rotating drum granulator is six feet in diameter and twenty feet long.
The slurry is added to the reactor and reacted with 8.6 gpm 99.5% ammonia, and an acid solution containing 8.6 gpm sulfuric acid (93%) and 2.6 gpm phosphoric acid (54% P2O5). The temperature of the reactor (due to the exothermic reaction betwe...
example 3
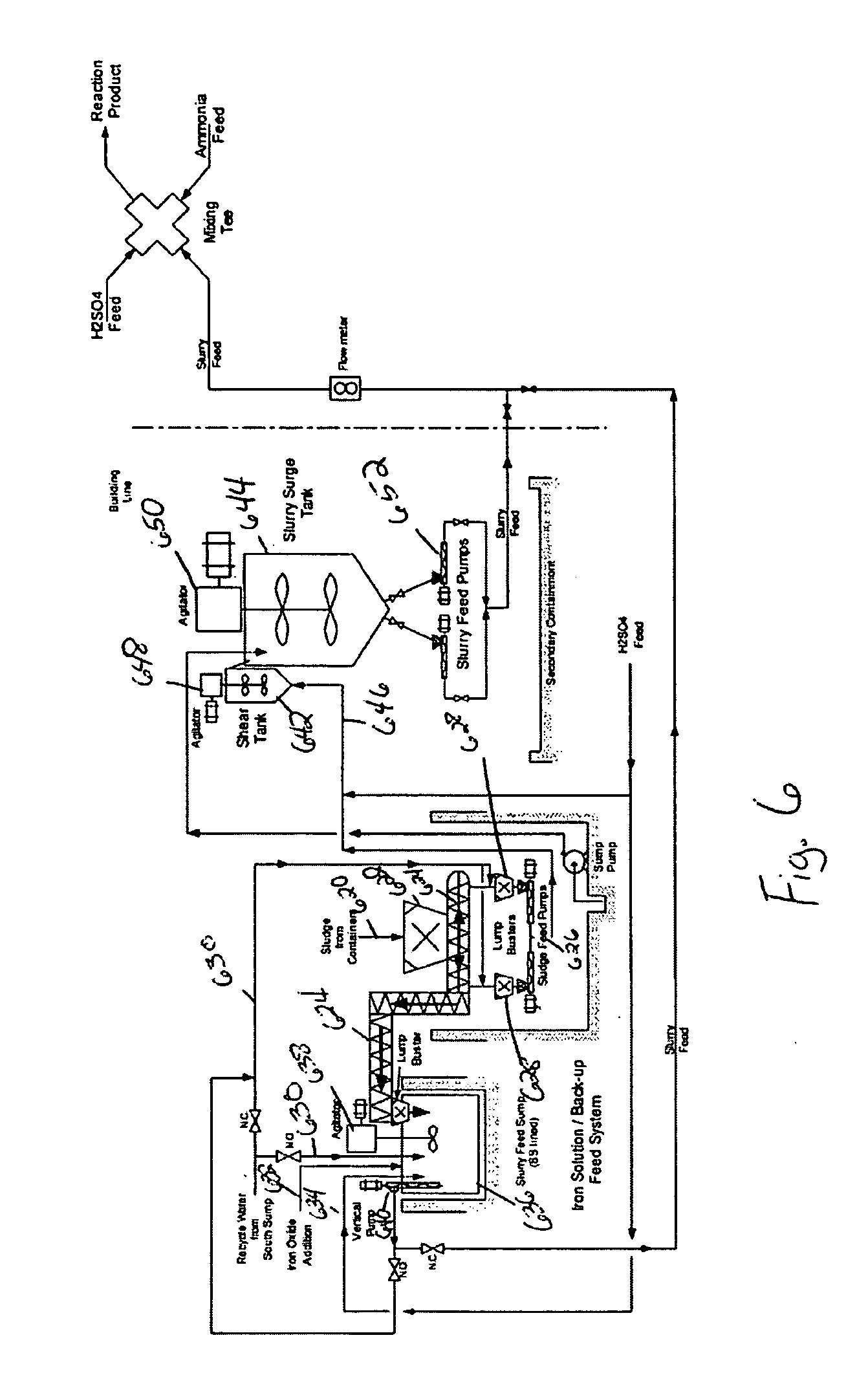
FIG. 6 shows one preferred method of preparing and handling the biosolids prior to their conversion into fertilizer.
In FIG. 6, municipal biosolids 620 are dispensed into 625 cubic yard boxes. These boxes are preferably placed on suitably designed dumping vehicles and transported to the sludge handling area. The boxes are opened and dumped into a receiving hopper 622. The receiving hopper 622 preferably has a minimum containment volume of about 47 cubic yards. The maximum volume of hopper 622 is dictated by the available space and general physical arrangement of the plant. The hopper 622 is preferably constructed of stainless steel to protect against corrosion as a result of the wet environment in the area. A large open grate is preferably installed inside the hopper to capture any large tramp materials that may be present in the sludge boxes. Preferably, this grating has openings of approximately 1′×1′.
Sludge 620 passes through the grating into the bottom of the hopper 622. At t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com