Recombinant human adenovirus 3, and preparation method and application thereof
An adenovirus and genome technology, applied in the field of human type 3 and type 7 adenovirus bivalent vaccine candidate strains and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Construction of recombinant virus vector
[0018] Shuttle vector construction: HAdv3-gz01 virus strain genome (Genbank accession no.DQ099432) was used as a template to PCR amplify the 26,782 to 27,736, 973bp E3L fragment (KpnI+ClaI) and the genome 30,900 To 31,901, 1020bp long E3R fragment (SpeI+NotI), use pEGFP C2 vector as a template to PCR amplify CMV-eGFP-SV40 expression cassette (ClaI+SpeI), and sequentially digest and ligate to pBluescript II SK(+) vector Above, the vector pSKE3LCMV-eGFP-SV40E3R (deleting the 3203 bp sequence of the E3 region) including the left and right arm sequences of the E3 pressure was obtained. With primer pair A7Hu(5'-aat cccggg ccaccatggccacccccatcgatgatgcc-3', AvaI site was introduced) and A7Hr (5'-taaaagcttttatgtggtggcgttgccg-3', HindIII site was introduced) PCR amplified HAdv7 hexon whole gene, AvaI+HindIII double digestion followed by AgeI+HindIIl double digestion The vector pSKE3LCMV-eGFP-SV40E3R was connected, and t...
Embodiment 2
[0021] Example 2: Identification of Capsid Composition of Recombinant Human Adenovirus Type 3 Particles
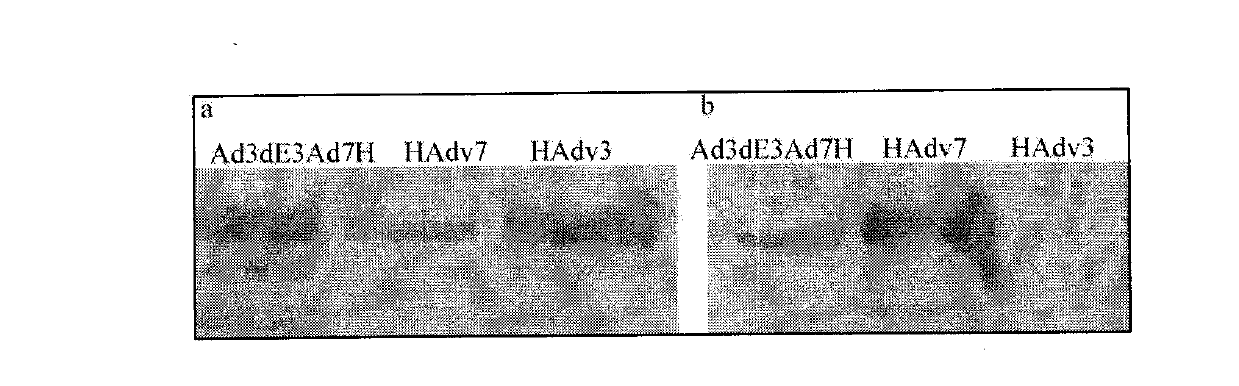
[0022] In order to verify the composition of the capsid hexon of the obtained recombinant virus particles, Western Blot detection was performed with HAdv3 and HAdv7 hexon type-specific antibodies. Antiserum immunized with Ad3-R4 epitope (VKPTTEGGVET) GST fusion protein mainly reacted with HAdv3 hexon, but only weakly reacted with HAdv7 hexon; antiserum immunized with Ad7-R4 epitope (VKPTEGDVET) GST fusion protein Only reacts with HAdv7 hexon, but not with HAdv3 hexon. Western Blot test results see figure 1 , figure 1a is the result of detection of anti-Ad3-R4-GST antiserum and purified Ad3dE3Ad7H virus particles, figure 1 b is the graph of detection results of anti-Ad7-R4-GST antiserum and purified Ad3dE3Ad7H virus particles. The results showed that the prepared and purified Ad3dE3Ad7H virus particles were chimeras containing two types of hexons, HAdv3 and HAdv7.
Embodiment 3
[0023] Example 3: Immunogenicity of Recombinant Viral Vectors and Recombinant Viral Particles
[0024] Antiserum was prepared and serum neutralization test was performed to verify its immunogenicity.
[0025] Recombinant virus vector: the purified Ad3dE3Ad7H virus vector was intramuscularly injected into mice (1×10 10 VPs / mouse), obtain polyclonal serum, and then carry out micro-neutralization test to detect whether the antibody produced by the recombinant virus vector has the ability to neutralize HAdv3 and HAdv7 viruses in vitro.
[0026] Recombinant virus particles: After inactivating the purified Ad3dE3Ad7H virus particles with β-propiolactone or formalin, immunize mice (1×10 10 VPs / mouse), obtain polyclonal serum, and then perform microneutralization test to detect whether the antibodies induced by recombinant virus particles have the ability to neutralize HAdv3 and HAdv7 viruses in vitro.
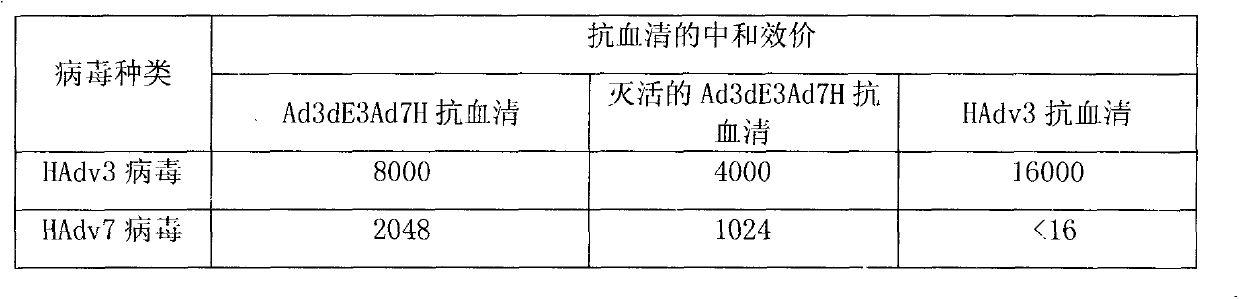
[0027] The results are shown in Table 1, which is the neutralization titer of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com