Recombinant adenovirus and tetravalent adenovirus vaccine and preparation method thereof
A technology of recombinant adenovirus and adenovirus, which is applied in the field of genetic engineering, can solve the problem of no prevention, and achieve the effect of similar genome copy number and replication efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 6
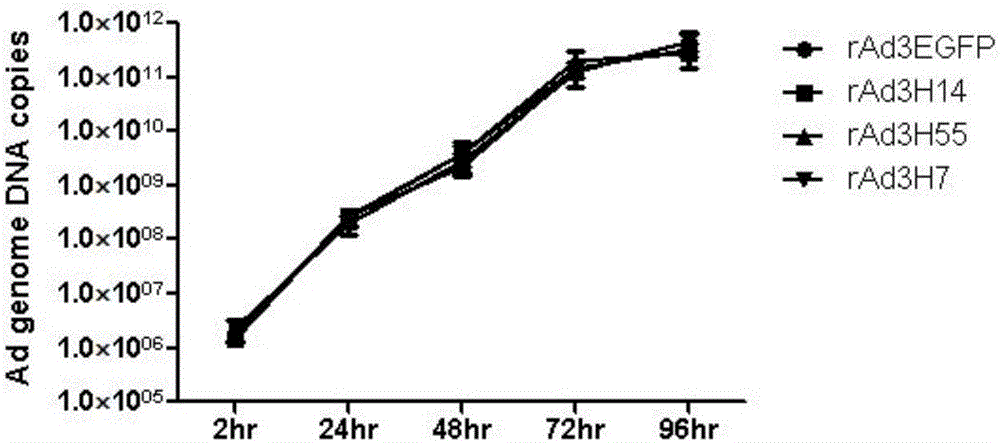
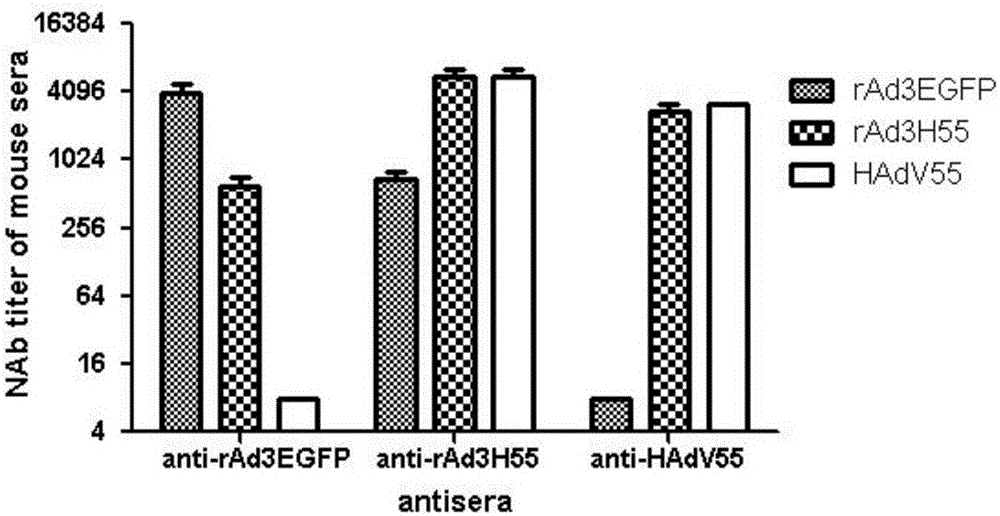
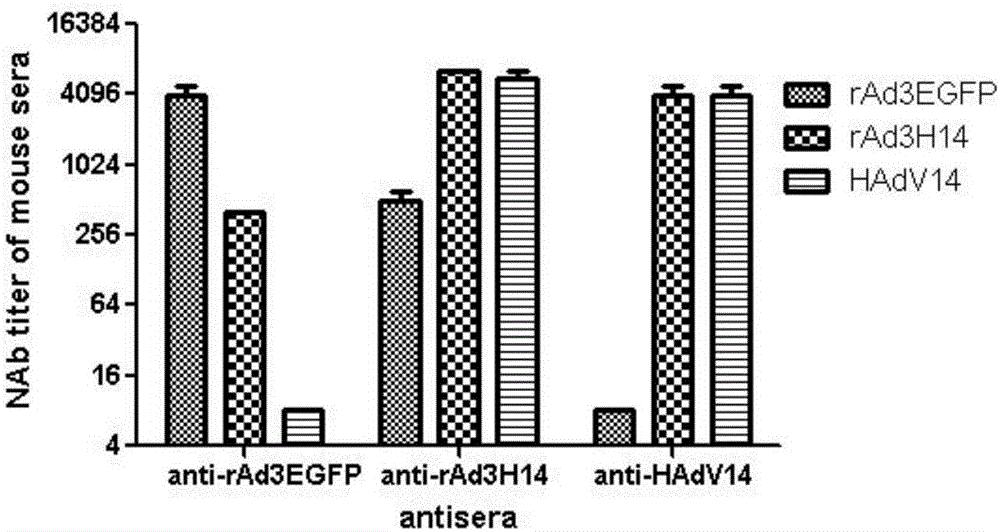
[0034] Example 1 Preparation of hexon replacement type recombinant adenovirus rAd3H14, rAd3H55 and rAd3H7
[0035] The backbone plasmid pBRAd△E3GFP obtained by the inventor in the early stage (published documents are Zhang Q, Su X, Seto D, Zheng BJ, Tian X, Sheng H, Li H, Wang Y, Zhou R. Construction and characterization of a replication-competent human adenovirus type 3-based vector as a live-vaccine candidate and a viral delivery vector. Vaccine 2009; 27(8):1145-53) as the basis, using the full-length hexon gene of Ad7, Ad14 or Ad55 (amplified from The virus strain of Ad7, Ad14 or Ad55, without mutation in the amino acid sequence) replaces the hexon gene of the recombinant type 3 adenovirus to obtain the hexon replacement type recombinant adenovirus rAd3H7, rAd3H14, rAd3H55. The basic construction procedure is as follows: amplify the hexon gene of Ad7, Ad14 or Ad55 by PCR, clone it into the hexon shuttle plasmid pBRHexonL / R, use pBRAd△E3GFP as the backbone plasmid, and use t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com