Genetically-engineered vaccine against fowl adenovirus type 4, and preparation method and application thereof
A technology of genetically engineered vaccine and poultry adenovirus, which is applied in the field of type 4 poultry adenovirus genetically engineered vaccine and its preparation, can solve the problems of harsh culture conditions, high production costs, and difficulty in large-scale cultivation, and achieve high protein yield and safe The effect of high sex and strong immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of the vaccine can adopt the conventional preparation method of genetic engineering subunit vaccine in the field.
[0041] Preferably, the preparation method includes:
[0042]The pMD19-T vector containing the target gene was double digested with Bam HI and Hind III to obtain the target fragment, and the target fragment was recovered and purified from the gel. At the same time, the pFastBac 1 vector was digested with double enzymes and recovered by gel. The target gene fragment and pFastBac 1 vector were ligated overnight with T4 DNA ligase. Then it was transformed into DH5α competent cells, picked and cultured and then sent for sequencing. After the sequencing is correct, extract the pFastBac 1 vector containing the target gene.
[0043] The pFastBac 1 vector containing the target gene was transformed into DH10Bac, and the transformed product was cultured in 37°C SOC medium for 5 hours, then diluted and inoculated on a blue-white spot screenin...
Embodiment 14
[0048] Example 1 Preparation of Type 4 Avian Adenovirus Genetic Engineering Vaccine
[0049] 1. Screening and cloning of target genes
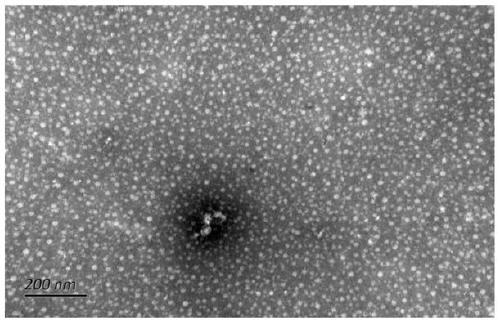
[0050] Find the protein coding gene sequence and protein sequence of penton and hexon of avian adenovirus, and perform sequencing comparison on RNA extracted from existing viruses. According to the previous research results, the region with strong antigenicity was selected for splicing, and a linker (Linker) was added between the penton and hexon proteins to ensure its spatial conformation and the formation of virus-like particles (VLPs).
[0051] Check the restriction enzyme cutting sites in the optimized sequence to ensure that it does not contain Bam H I and Hind III cutting sites, and send the spliced sequence to the biological company for synthesis.
[0052] A pair of splicing primers for amplifying avian adenovirus gene fragments were designed with Primer5.0 software, and protective bases and restriction sites were added to the 5' end...
Embodiment 2
[0064] Example 2 Expression characteristics and immunogenicity of type 4 avian adenovirus genetic engineering vaccine
[0065] 1. Expression characteristics
[0066] Sf9 cells were cultured to 5 × 10 in Sf9 serum-free suspension medium 6 cells / ml, dilute the cell density to 2.5×10 with fresh serum-free medium 6 cells / ml, the recombinant baculovirus was inoculated at a ratio of 1‰, and the supernatant was harvested after culturing for 96 hours. The cells at 96h post-infection were significantly larger than the healthy cells. Determine virus titer by plaque method, recombinant baculovirus P1 generation virus titer 10 8.25 TCID 50 / ml.
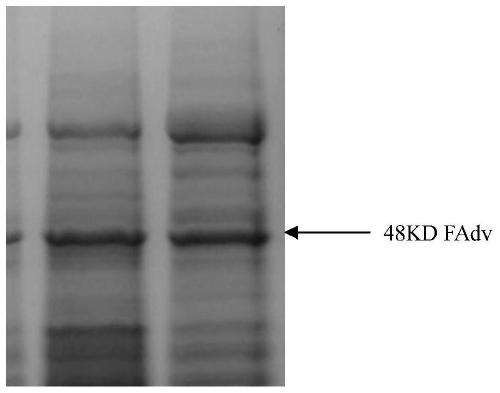
[0067] Sf9 cells were cultured with Sf9 serum-free suspension medium to 5 × 10 6 cells / ml, dilute the cell density to 2.5×10 with fresh serum-free medium 6 cells / ml, inoculate the recombinant baculovirus at a ratio of 1%-1‰, culture until 168-192h and harvest the culture supernatant. Carry out SDS-PAGE electrophoresis identification prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com