CMV virus-like particle for producing VLP recombinant vaccine and preparation method thereof
A recombinant vaccine, virus-like technology, applied in the fields of virology, molecular biology, immunology and medicine, which can solve the problems of inability to achieve high-efficiency modification at sites, low modification efficiency of chemical linkers, and inhomogeneity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 of the present invention provides a CMV virus-like particle for the production of a VLP recombinant vaccine. The CMV virus-like particle clones the C-terminal G4SLPETG-modified plant virus cucumber mosaic virus CMV capsid gene into a prokaryotic expression vector to obtain a recombinant expression vector , the recombinant expression vector was transfected into Escherichia coli BL21(DE3), and obtained through expression of the recombinant Escherichia coli BL21(DE3); the amino acid sequence of the CMV modified with G4SLPETG at the C-terminus is SEQ ID NO.1.
Embodiment 2
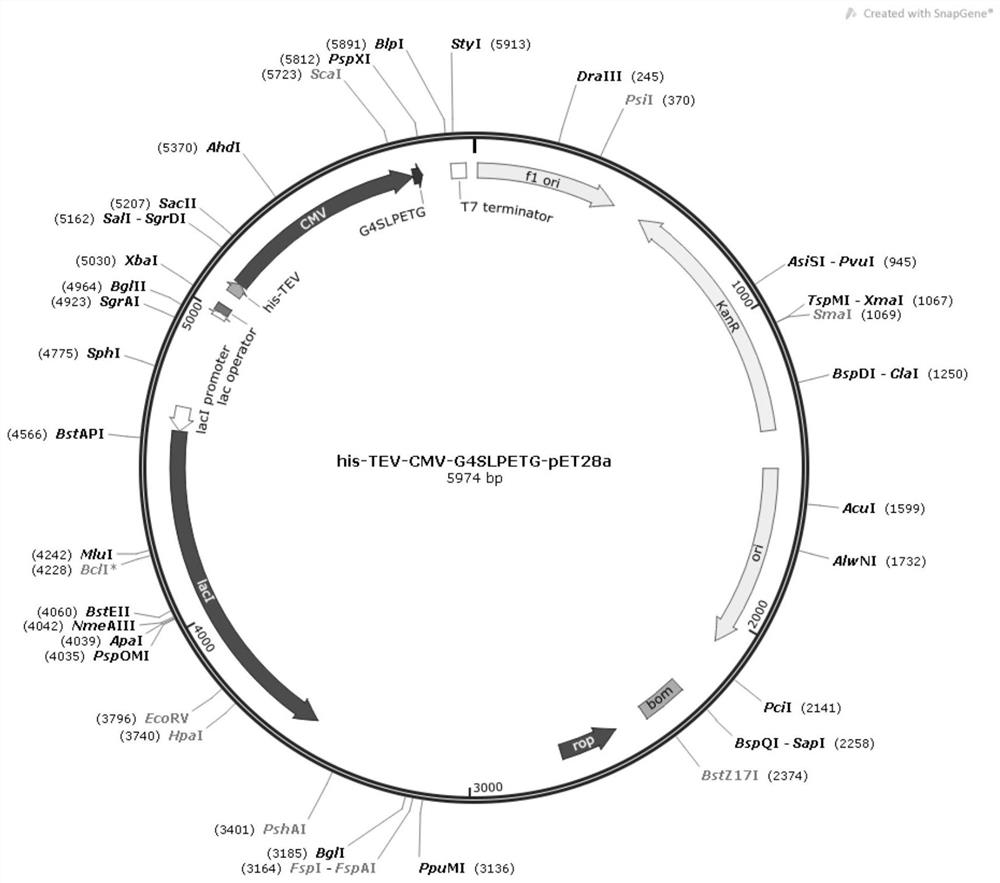
[0030] see figure 1 and figure 2 Embodiment 2 of the present invention provides a method for preparing CMV virus-like particles for producing VLP recombinant vaccines, comprising the following steps:
[0031] (1) Construction of the recombinant plasmid: the CMV-G4SLPETG gene was directly synthesized by BGI into the pET28a vector NdeI restriction site and XhoI restriction site to obtain the recombinant plasmid his-TEV-CMV-G4SLPETG-pET28a;
[0032] (2) Transfer the recombinant plasmid into the expression strain: transfer the his-TEV-CMV-G4SLPETG-pET28a recombinant plasmid into the Escherichia coli BL21 (DE3) expression strain to obtain the recombinant expression strain his-TEV-CMV-G4SLPETG-pET28a-BL21;
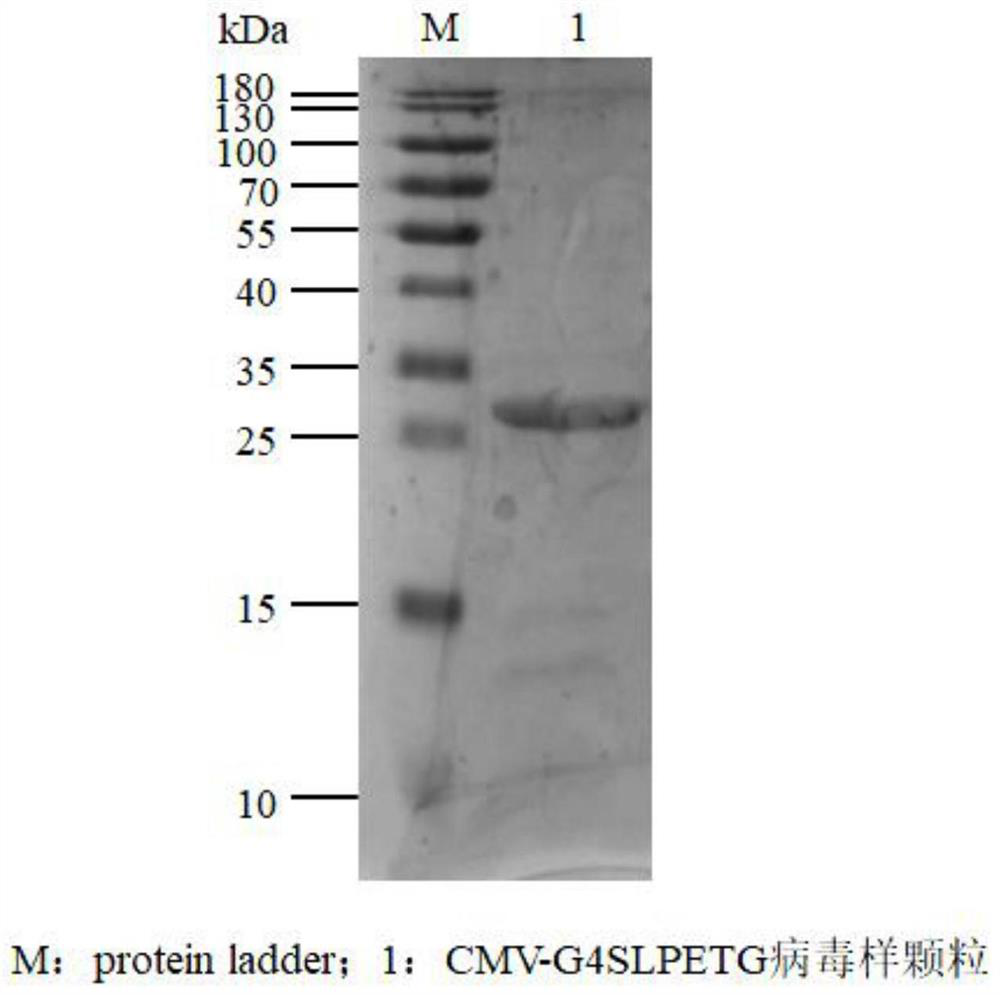
[0033] (3) Bacterial culture and purification of his-TEV-CMV-G4SLPETG virus-like particles: culture the recombinant expression strain his-TEV-CMV-G4SLPETG-pET28a-BL21, and induce expression with IPTG to obtain his-TEV-CMV-G4SLPETG virus-like particles.
[0034] (4) Preparation ...
Embodiment 3
[0046] Example 3 of the present invention provides a VLP recombinant vaccine, which is prepared by mixing the CMV virus-like particles of Example 1 and the target antigen at a molar ratio of 1:1, and then catalyzed by Sortease A, which simplifies the production of the vaccine.
[0047] Specifically, the CMV-G4SLPETG virus-like particle in Example 1 and the target antigen modified by three glycines at the N-terminus were prepared in PBS (10 mM) to make 1 mg / ml respectively, mixed at a ratio of 1:1, and his-Sotase A5 enzyme was added, According to the proportion of 100ug catalyzed 2mg mixed protein, shake and catalyze at 37°C for 5h, then pass the catalytic solution through the nickel column, the penetration solution is CMV-G4SLPETGGG-target antigen virus-like particles, and his-Sotase A recombinase will hang on the nickel column , using a 3K concentration tube to concentrate the penetrating fluid, ie, CMV-G4SLPETGGG-target antigen virus-like particles, to obtain a VLP recombinan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com