Recombined bifidobacteria hRV-VP4 expression vector and oral vaccine thereof
A technology of bifidobacteria and expression vector, applied in the field of genetic engineering, can solve the problems such as no report of bifidobacteria, and achieve the effect of high safety and broad clinical application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Construction of Bifidobacterium-Escherichia coli shuttle expression vector of the present invention
[0035] 1. replace the Ptac promoter (183-932) on the pGEX-5x-1 with the promoter AmyO (position: 599-690nt) of the α-amylase (amylase) gene (GenBank No: AY240946) of bifidobacteria, and simultaneously The LacIq fragment (position: 3301-4420nt) on pGEX-5x-1 was deleted by PCR method.
[0036] Specific steps are as follows:
[0037] 1. Using the pGEX-5x-1 plasmid as a template, use high-fidelity Taq DNA polymerase to obtain the basic skeleton of the vector by PCR amplification.
[0038] PCR primer F is the multiple cloning site (MCS) sequence on pGEX-5x-1, and primer R is the upstream sequence of LacIq. is the primer sequence for deleting LacIq on pGEX-5x-1.
[0039] The primer sequences are as follows: pF1: ggatcc ccgaattcccg (SEQ ID NO.4) (position: pGEX-5x-1934-949, containing the added BamHI restriction site);
[0040] pR1: ccgcgg attcaccaccctgaa...
Embodiment 2
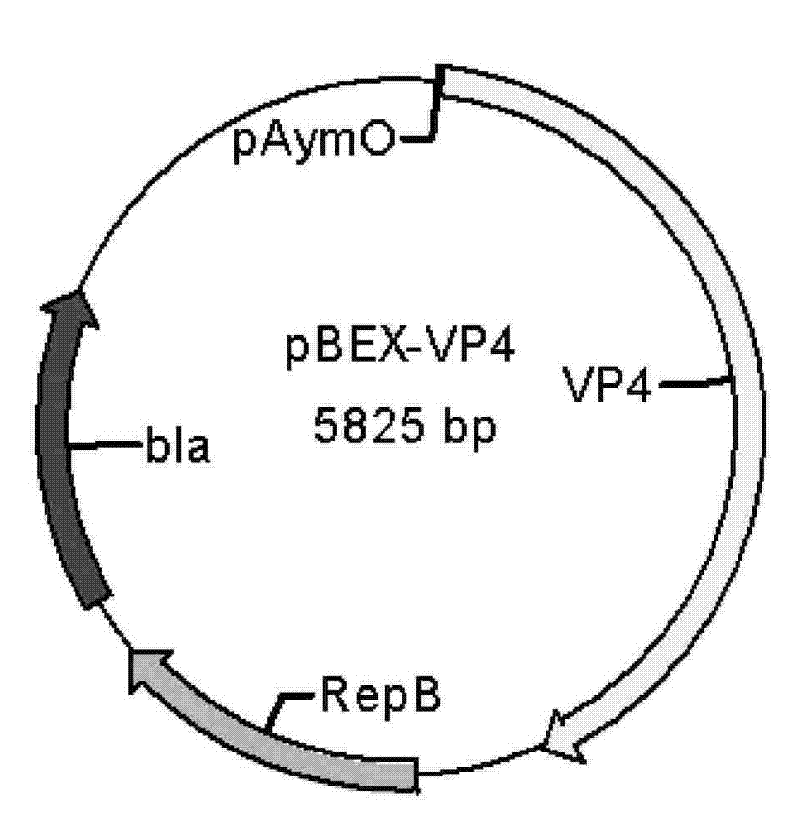
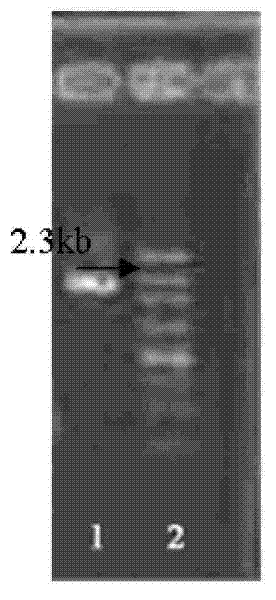
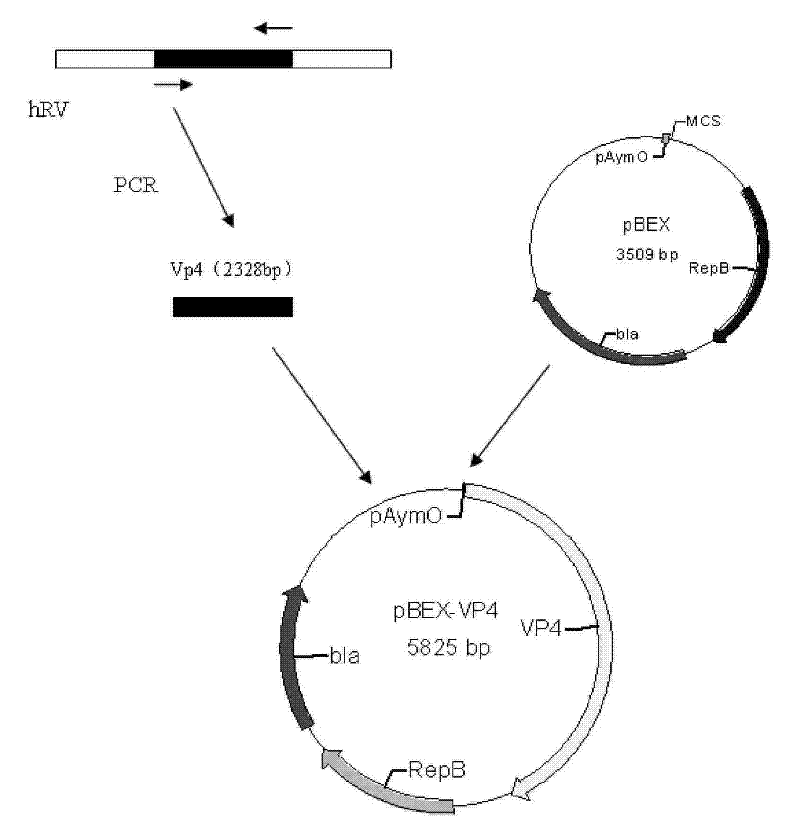
[0062] Embodiment two constructs pBEX-hRV VP4 expression vector (process schematic diagram sees attached image 3 )
[0063] 1. Amplify the viral protein VP4 gene (GENBANK NO.L34161, coding position: 10-2337nt, as shown in SEQ ID NO.2) from the genome of Wa strain hRV virus by RT-PCR method.
[0064] Primers are as follows:
[0065] Vp4F: ggatccggctataaaatggcttcact (SEQ ID NO. 13) (BamHI site added).
[0066] Vp4R: gtcgaccacatcctcaatagcgttct (SEQ ID NO. 14) (SalI site added).
[0067] Methods as below:
[0068] Take 10 μl of extracted RNA, heat at 100°C for 2 minutes to denature the double-stranded RNA, and then centrifuge briefly after 2 minutes on ice, then add 4 μl of 5×AMV Bufer, 2 μl of dNTPs, 1 μl of downstream primer (30 μM / μl), 1 μl of RNasin, and reverse Transcriptase AMV 2μl, add ddH20 to make up the 25μl reaction system.
[0069] Reverse transcription at 42°C for 1 hour. Take a clean and sterilized PCR tube and add to the 50 μl reaction system: 5 μl of 10×PCR ...
Embodiment 3
[0071] Embodiment 3 The preparation of the bifidobacterium seed bacteria containing pBEX-hRV-VP4 recombinant vector of the present invention
[0072] The procedure for transforming bifidobacteria is as follows:
[0073] 1. Select Bifidobacteria with an OD value of 0.6 as the recipient bacteria. After being treated with sterilized 10% glycerol prepared in deionized water, the plasmid vector is transformed into the recipient bacteria by electroporation; the transformation conditions are: 15KV, 200Ω , 25μF.
[0074] 2. Resuscitate the transformed bifidobacteria in MRS liquid medium without antibiotics for 60-120 minutes, and then culture them on MRS solid medium containing 50 μg / ml ampicillin to select resistant colonies to obtain transformed hRV - the bifidobacterium of VP4 gene, the positive clone after PCR and SDS-PAGE identification is the bifidobacterium seed fungus containing the pBEX-hRV VP4 recombinant vector.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com