A kind of anti-new coronavirus sars-cov-2 neutralizing nanobody and its application
A nanobody and coronavirus technology, applied in the direction of antiviral agent, virus/bacteriophage, antiviral immunoglobulin, etc., can solve the problem that there is no report of neutralizing nanobody against the new coronavirus SARS-CoV-2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Screening of nanobodies recognizing the RBD domain of the SARS-CoV-2 spike protein
[0080] Step 1: Rescue the phage surface-displayed nanobody library
[0081] The fully synthetic nanobody library is stored in the host bacteria in the form of phagemids. Before the panning process begins, the library is rescued to make it a phage-displayed antibody library. The specific method is as follows:
[0082] Take 1mL (OD 600 =100) antibody library Glycerol bacteria, inoculated into 5 bottles of 200mL 2TY-CARB medium (OD 600 =0.1), 37°C, 250rpm shake to OD 600 = about 0.5; add 1.6×10 12 PFU M13KO7, let stand at 37°C for 30 minutes, shake the bacteria at 37°C at 200rpm for 30 minutes; transfer the bacterial liquid to a centrifuge bottle, centrifuge at 2200g for 15 minutes, resuspend the bacteria with 400mL 2TY-CARB-KAN, 30°C, 250rpm Shake the bacteria for 14-16 hours; divide the bacteria solution into centrifuge bottles (200mL per bottle), centrifuge at 10000g, 4°...
Embodiment 2
[0091] Example 2: Fusion expression of nanobody and Fc protein
[0092] Step 1: Construction of recombinant expression vector
[0093] The positive cloned gene was amplified using the upstream primer shown in SEQ ID No.8 and the downstream primer shown in SEQ ID No.9.
[0094] SEQ ID No.8: 5'-ggcgctagccaagttcaattggttgaat-3'
[0095] SEQ ID No.9:
[0096] 5'-tgagcctccactgaattcagaagaaacagtaacttgagtacct-3'.
[0097] A nucleic acid molecule encoding an anti-RBD Nanobody is obtained. The antibody gene was digested with NheI and EcoRI, inserted into the antibody expression vector pCDNA4-Fc, and transformed into DH5a Escherichia coli competent cells (purchased from Quanshijin Company) to obtain recombinant bacteria. The recombinant bacteria were inoculated in liquid LB-Amp medium, cultivated overnight at 37°C, and the plasmid was extracted with a plasmid extraction kit. 16 nanobody recombinant plasmids were obtained. pCDNA4-Fc was transformed from pCDNA4 / myc-HisA (purchased fro...
Embodiment 3
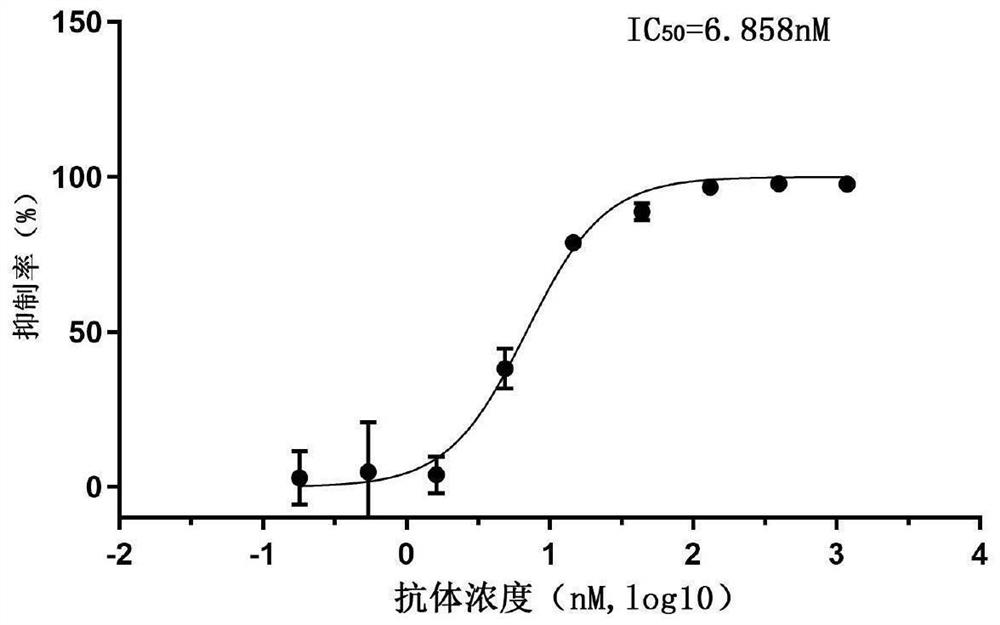
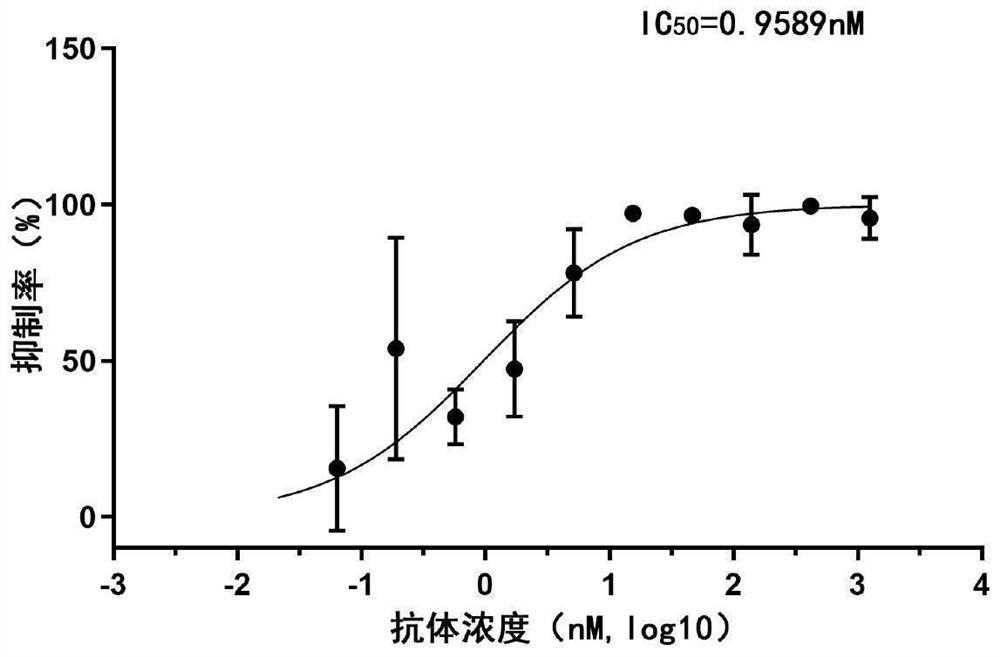
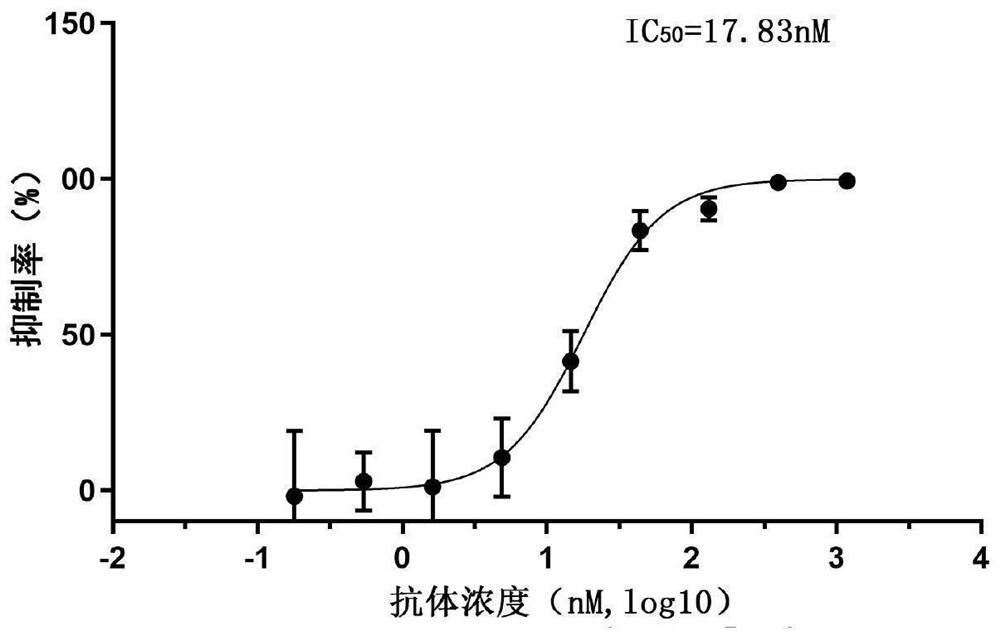
[0100] Example 3: SARS-CoV-2 pseudotyped virus neutralizing activity assay of Nanobodies
[0101] Step 1: SARS-CoV-2 pseudotyped virus packaging
[0102] 6×10 6 HEK293T cells (from the Cell Resource Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences) were inoculated in a 10cm culture dish, and the medium used was DMEM high-glucose medium (purchased from Thermo Fisher) containing 10% fetal bovine serum (purchased from Thermo Fisher). From Thermo Fisher), after 12 hours, transfect 10 μg S gene expression plasmid (Jinweizhi) and 10 μg pNL4.3-Luc-R-E-plasmid (BioVectorNTCC), and replace with 2% fetal bovine serum 12 hours after transfection DMEM high-glucose medium, continue to cultivate for 48 hours and harvest the culture supernatant containing SARS-CoV-2 pseudotyped virus, aliquot and freeze at -80°C.
[0103] Step 2: SARS-CoV-2 pseudotype virus invasion inhibition experiment
[0104] 10 4 Calu-3 cells (from the Cell Resource Center, Institute ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com