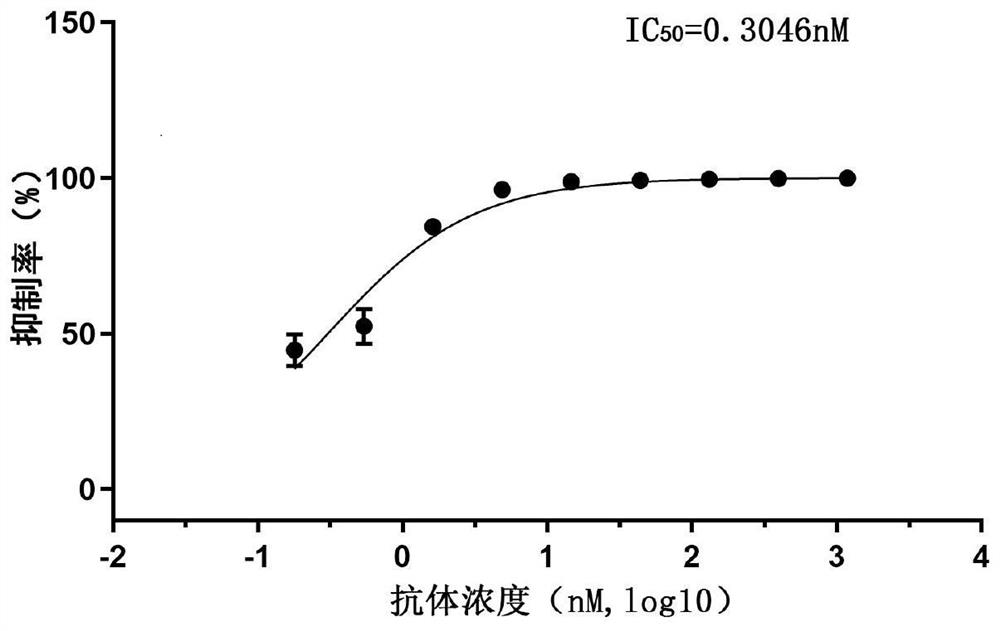
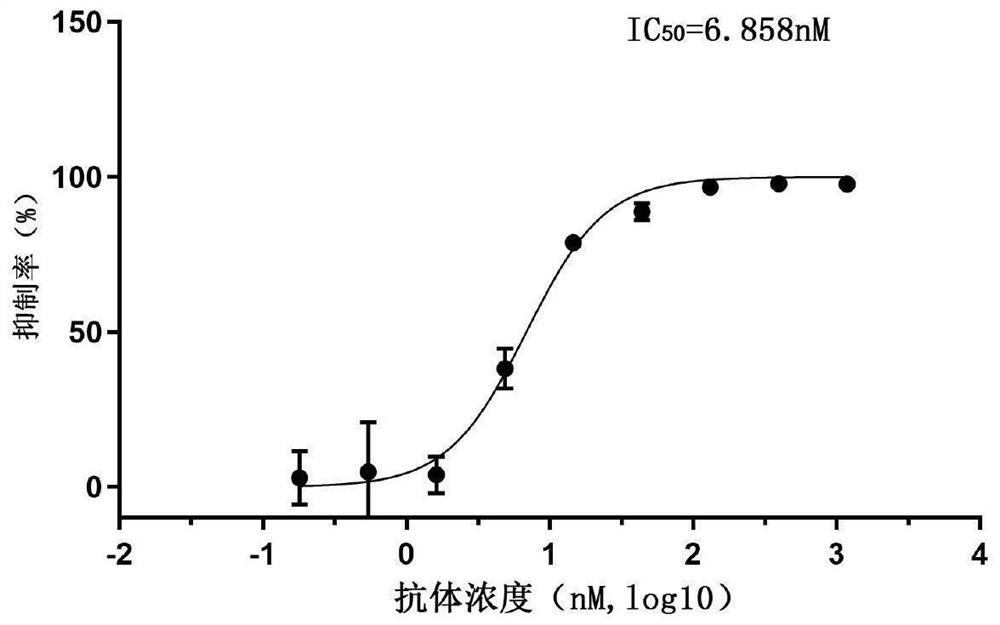
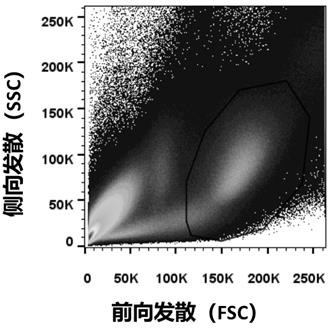
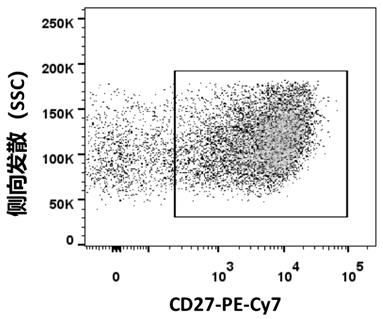
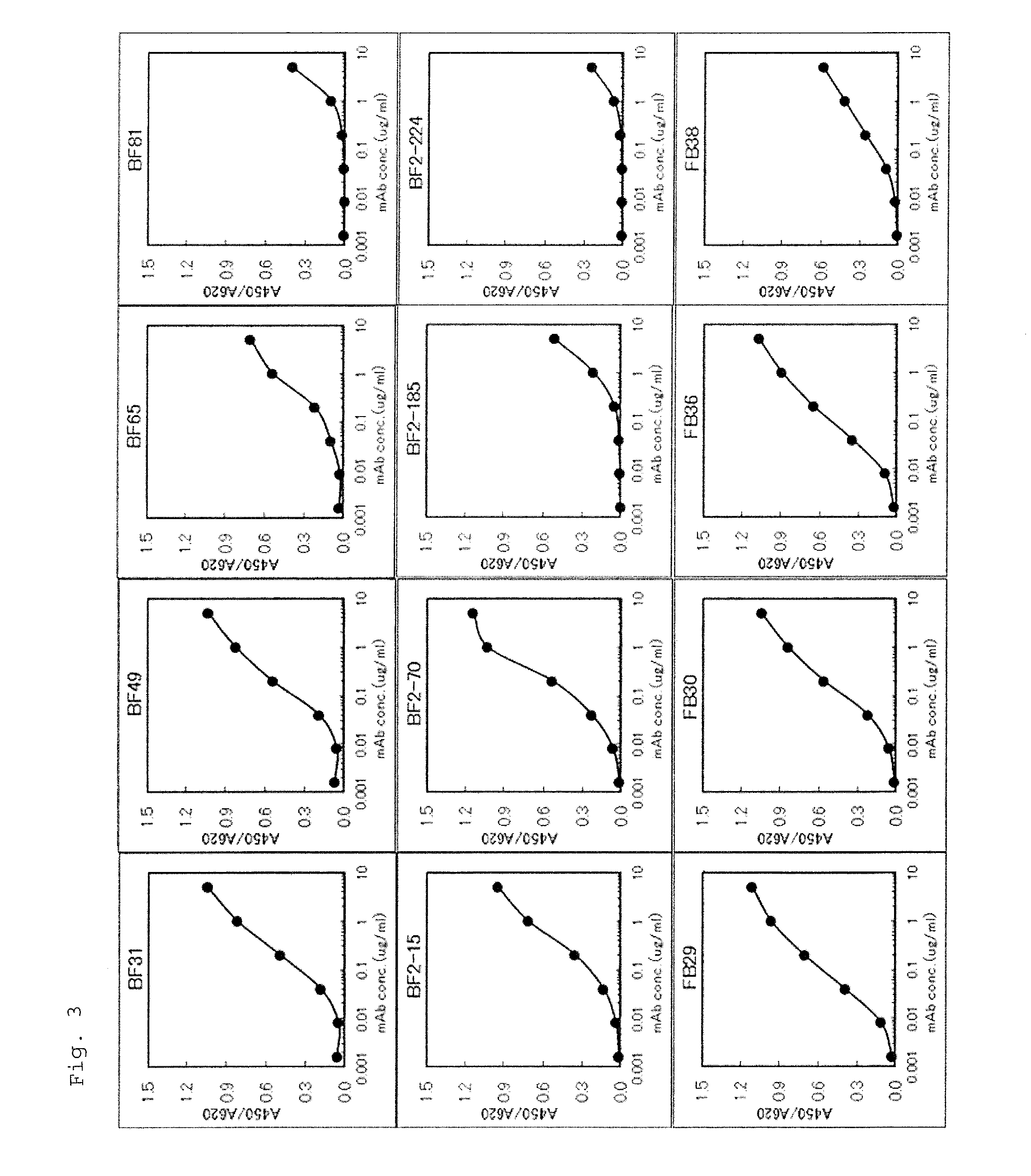
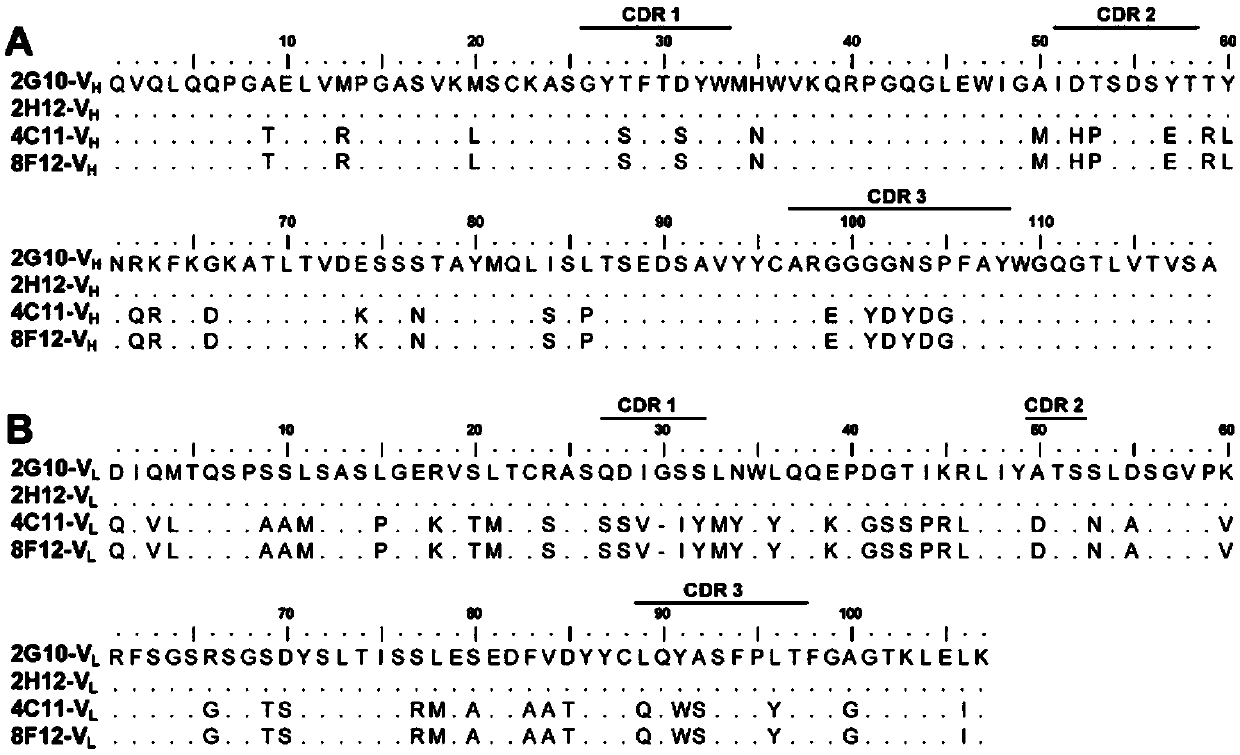
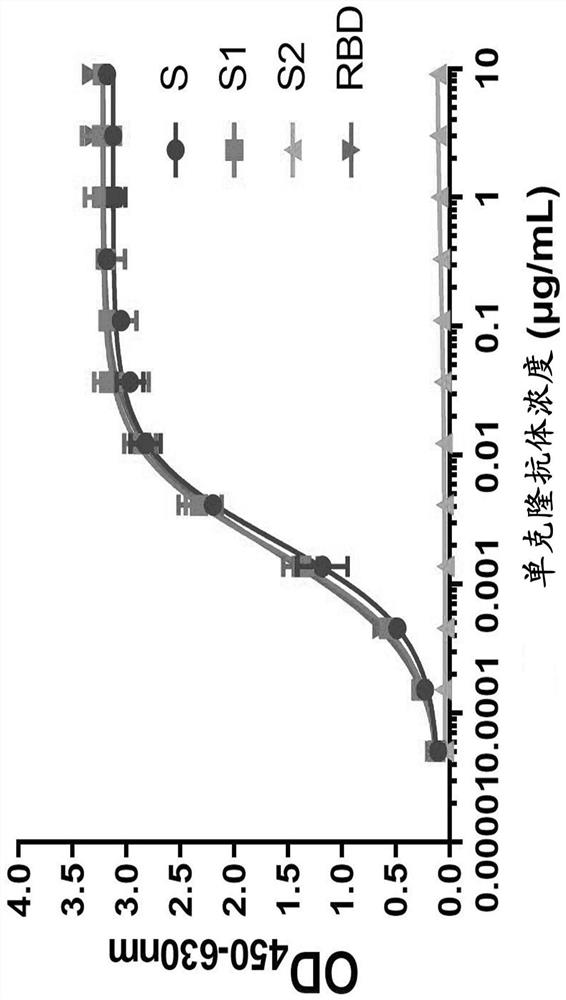
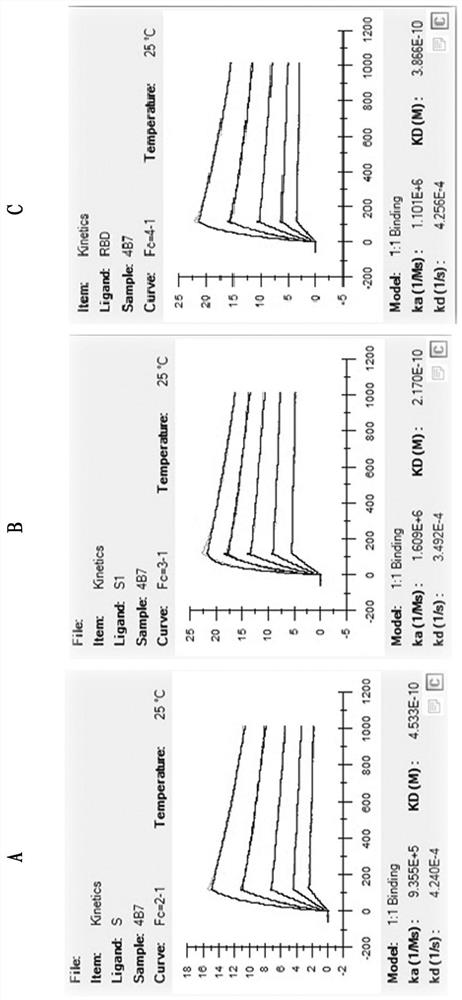
Patents
Literature
56results about How to "High neutralizing activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monoclonal antibody and antibody composition for neutralizing canine distemper virus (CDV)
ActiveCN103059133AHigh neutralizing activityAvoid monotonyImmunoglobulins against virusesAntiviralsEpitopeCanine distemper virus CDV
The invention relates to a monoclonal antibody and an antibody composition for neutralizing canine distemper virus (CDV), belonging to the technical field of biology. The monoclonal antibody 2G3 disclosed by the invention has high activity for neutralizing CDV and is used for identifying epitopes differently acting on CDV with a monoclonal antibody for neutralizing CDV prepared from another screened hybridoma cell 1D7 strain. Two monoclonal antibodies are prepared into the antibody composition, the neutralizing capability on CDV of which is obviously prior to the single monoclonal antibodies 2G3 and 1D7 in the composition; after being combined, the two monoclonal antibodies can generate antiviral synergistic enhancement action; and both the range and the capability for neutralizing CDV are increased. The monoclonal antibody disclosed by the invention is used for preparing the antibody composition for treating clinical canine distemper paroxysm animal; the virus neutralizing capability is stronger; failure of the monoclonal antibody caused by CDV heteromorphosis can be avoided; and the clinical application range is wider.
Owner:JIANGSU ACAD OF AGRI SCI
Coxsackie virus A16 type virus-like particle vaccine
InactiveCN102465144AHigh neutralizing activityAntiviralsViruses/bacteriophagesCoxsackievirus a16Spatial configuration
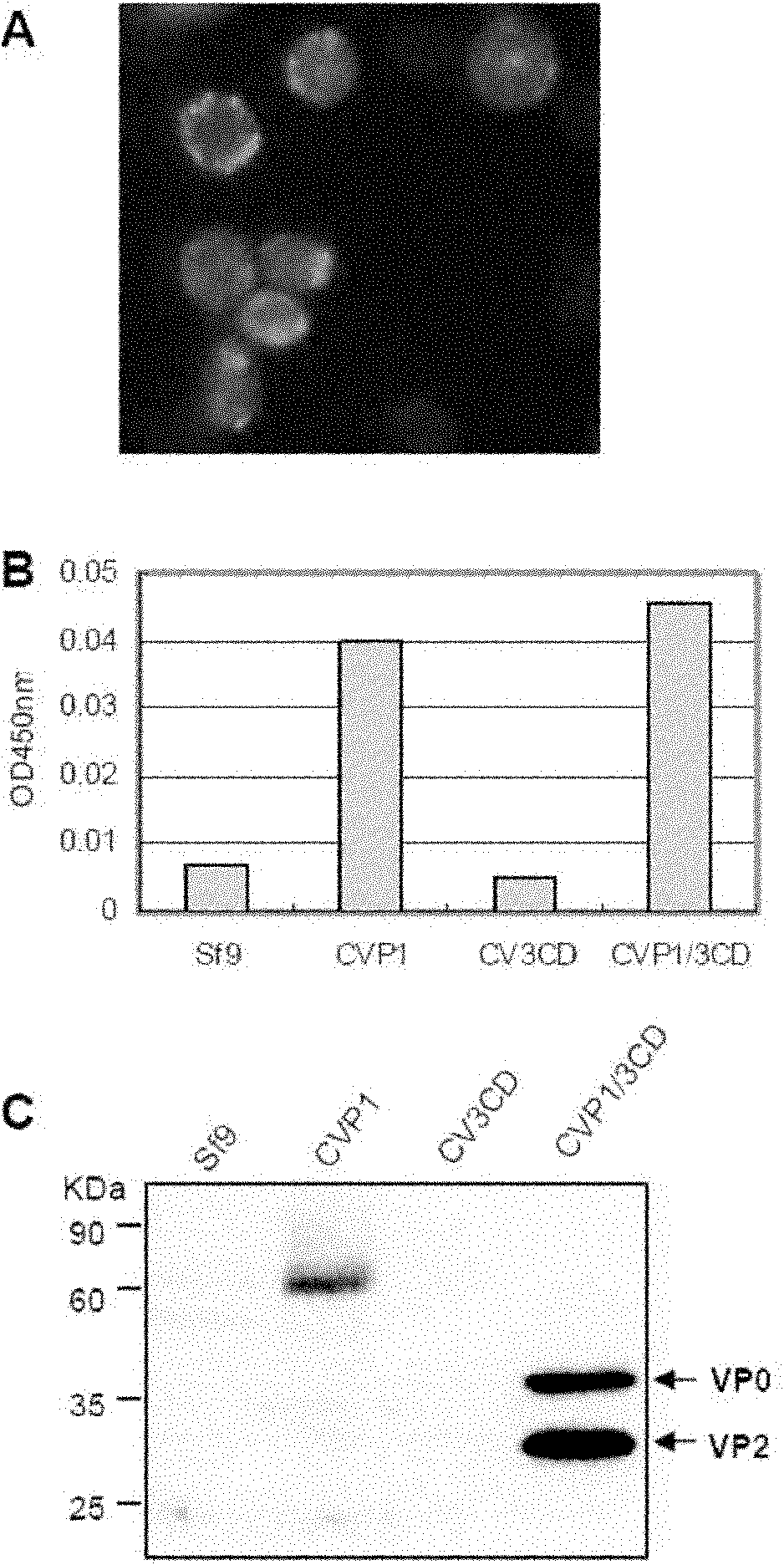
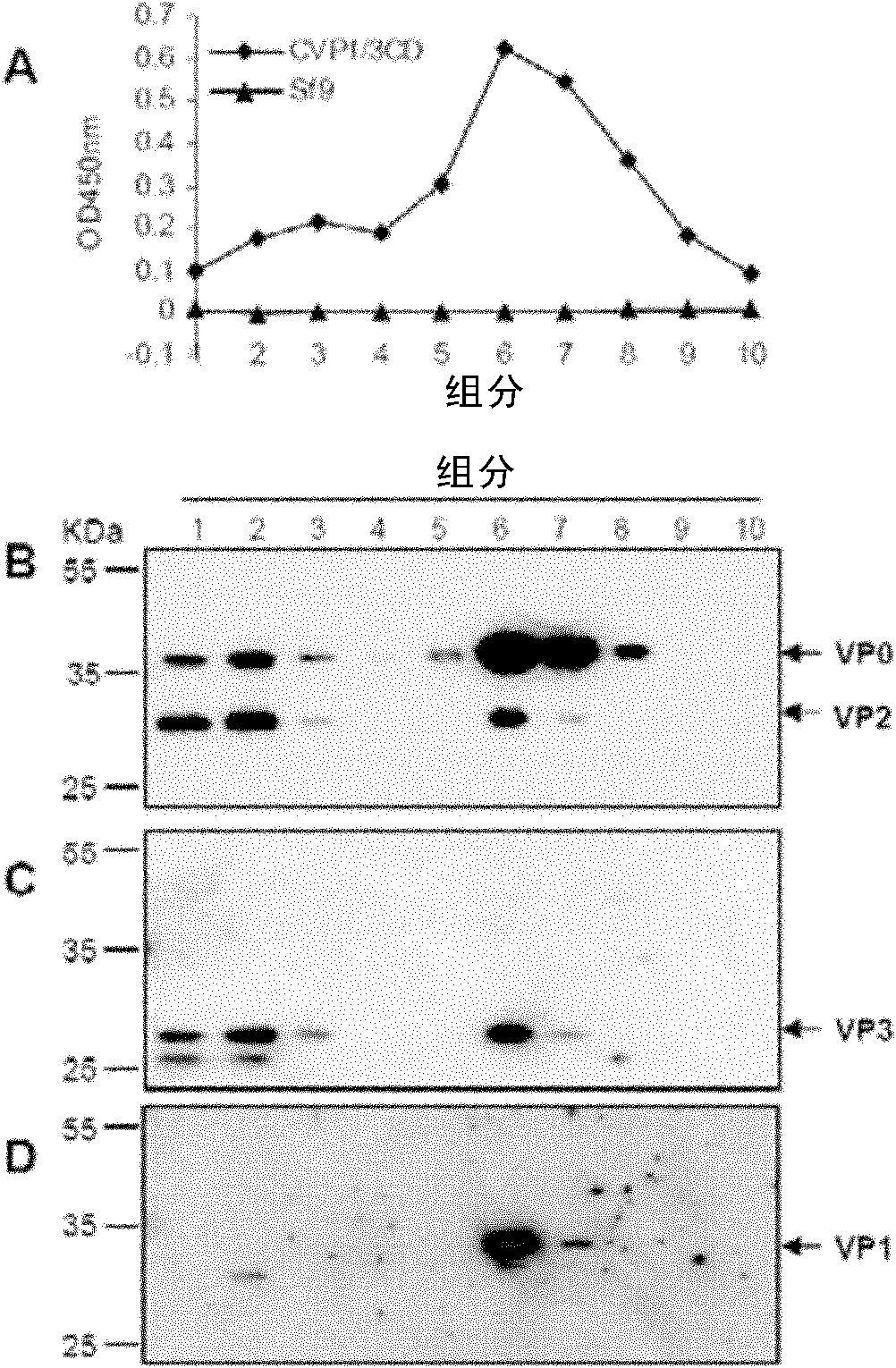
The invention relates to a coxsackie virus A16 type virus-like particle vaccine. The inventor fortuitously finds that proteins which have better spatial configurations and are suitably cut can be obtained by expressing P1 protein of CVA16 and 3CD protein of CVA16 by infecting insect cells with rhabdovirus. The proteins can be automatically assembled into a virus-like particle which has high immunogenicity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Neutralizing nano antibody for resisting novel coronavirus SARS-CoV-2 and application thereof
ActiveCN113563463AHigh neutralizing activityNot infectiousAntibody mimetics/scaffoldsGenetically modified cellsAntigenBiomedical engineering
The invention discloses a neutralizing nano antibody for resisting novel coronavirus SARS-CoV-2 and application thereof, and belongs to the technical field of biological medicine. Variable regions of the neutralizing nano antibody are provided with three complementary determining regions CDR1, CDR2 and CDR3; and the neutralizing nano antibody is any one of (a1)-(a6). The neutralizing nano antibody for resisting novel coronavirus SARS-CoV-2 provided by the invention can be effectively combined with an RBD antigen, can effectively compete with human ACE2 protein to be combined with the RBD antigen after being fused with a human IgGFc tag, has high neutralizing activity on SARS-CoV-2 pseudotype virus, and has important scientific significance and application prospects for prevention and clinical treatment of diseases caused by the novel coronavirus SARS-CoV-2 and research and development of diagnostic reagents.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Fully human monoclonal neutralizing antibody for resisting novel coronavirus and application of fully human monoclonal neutralizing antibody
ActiveCN112794899AAvoid infectionHigh neutralizing activityImmunoglobulins against virusesAntiviralsDiagnosis treatmentMutant strain
The invention relates to the field of biological medicine, and discloses a fully human monoclonal neutralizing antibody for resisting novel coronavirus and application of the fully human monoclonal neutralizing antibody. B-type lymphocytes are screened from blood of a new coronal pneumonia rehabilitation patient, and the affinity constant of the obtained antibody and a virus receptor binding domain (RBD) is 1.19 nM. X-ray crystal diffraction is used for analyzing the three-dimensional structure of the antibody and RBD compound, it is found that the binding epitope of the antibody and the binding epitope of a host cell receptor (ACE2) are highly overlapped, binding of the RBD and the ACE2 can be competitively blocked, and infection of the RBD and the ACE2 is inhibited. The antibody can cope with certain genetic mutations of viruses, the half effective concentration of the antibody for blocking pseudoviruses (accumulated B.1. 1.7 mutant strain genes and D614G) from infecting host cells is 0.57 ng / mL, and the antibody has the advantages of safety, high efficiency and broad spectrum, and is expected to be used as a neutralizing antibody drug for diagnosing, treating and preventing new coronal pneumonia.
Owner:SUZHOU YUZHIBO BIOLOGICAL TECH CO LTD
Bispecific antibody for resisting novel coronavirus and application of bispecific antibody
ActiveCN113563475AHigh neutralizing activityStrong inhibitory activityHybrid immunoglobulinsImmunoglobulins against virusesBispecific antibodyPharmaceutical drug
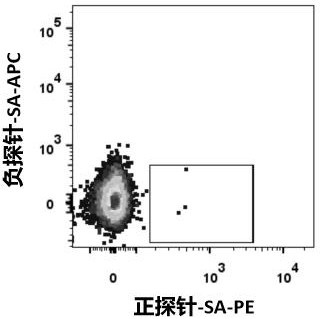
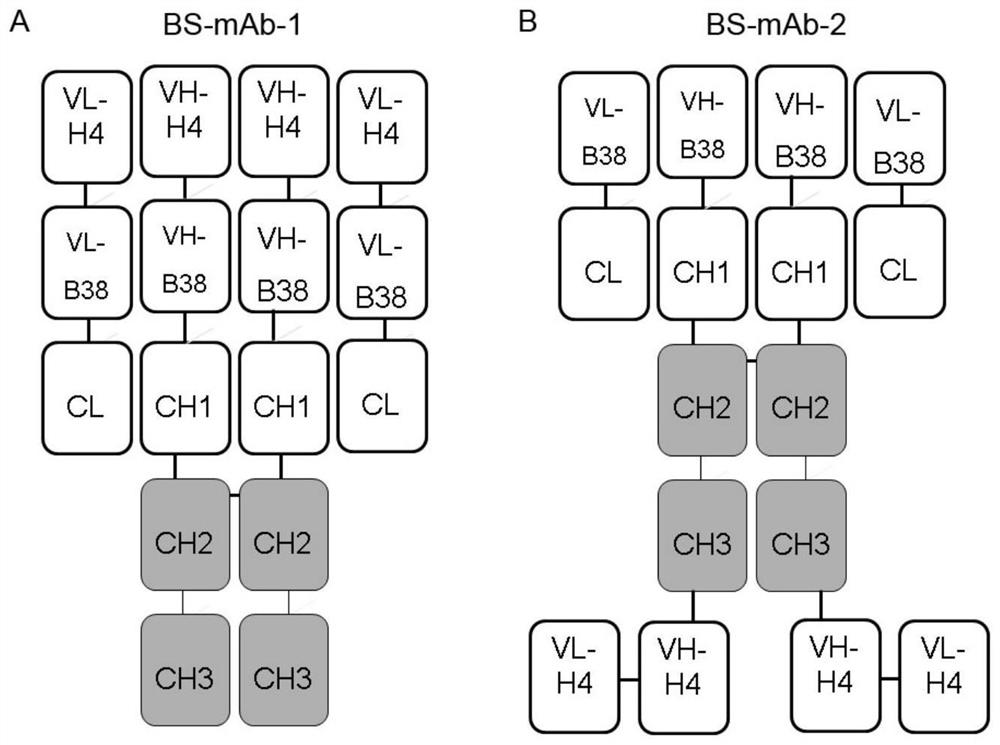
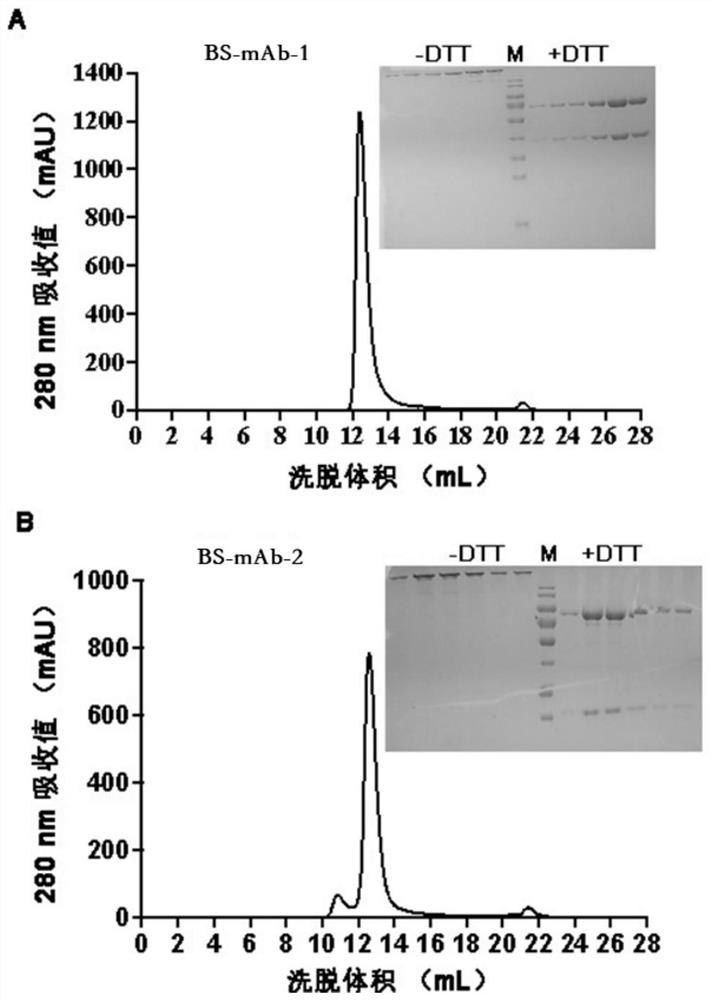
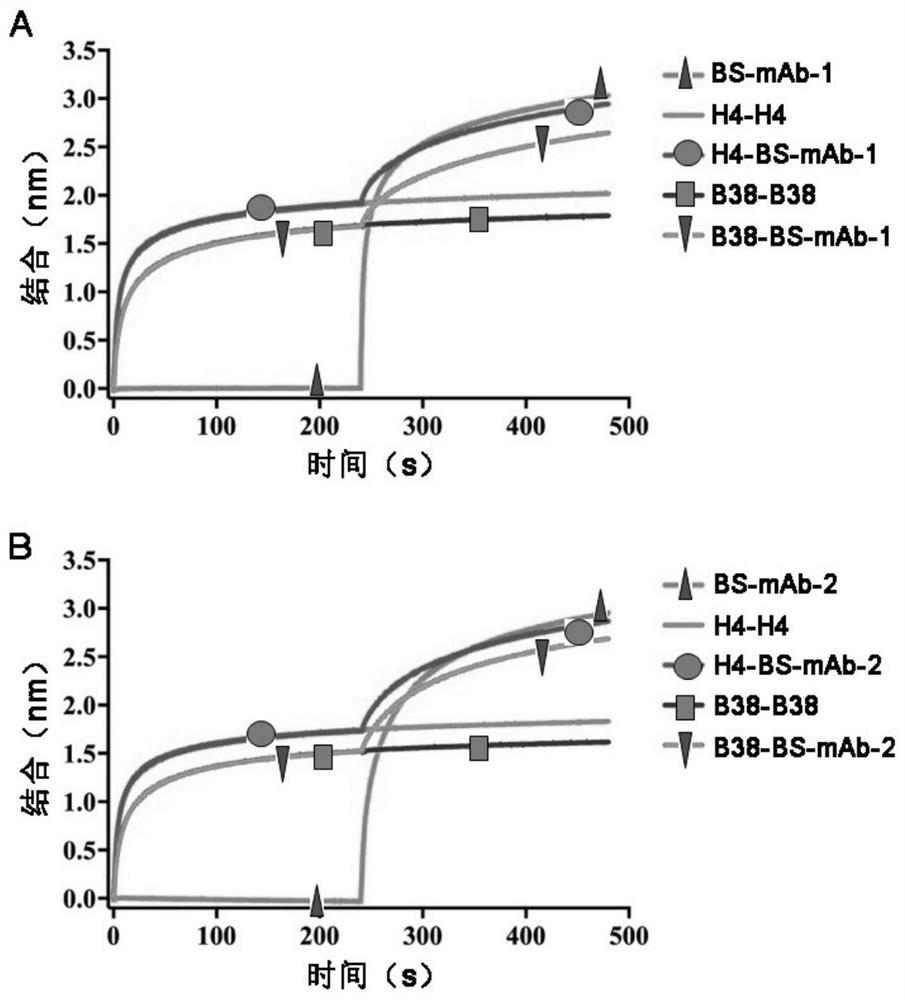
The invention relates to a bispecific antibody for resisting novel coronavirus and application of the bispecific antibody. The bispecific antibody is obtained by modifying new coronavirus monoclonal antibodies H4 and B38 through a genetic engineering method, different sites of novel coronavirus S protein RBD can be recognized at the same time, the neutralizing activity on new coronavirus pseudovirus is far higher than that of a maternal monoclonal antibody, and the inhibitory activity on new coronavirus live virus is further higher than that of the maternal monoclonal antibody. According to the bispecific antibody for resisting novel coronavirus, the selectivity and neutralizing activity of the maternal monoclonal antibody are improved, and the safety and effectiveness of monoclonal antibody drugs are improved; and the bispecific antibody can be used for preparing potential drugs for diagnosing, preventing and treating diseases caused by novel coronavirus and is huge in market value and good in application prospect.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Monoclonal antibody of novel coronavirus and mutant thereof and application of monoclonal antibody
ActiveCN113943368AHigh affinityHigh neutralizing activityImmunoglobulins against virusesAntiviralsAntigenComplementarity determining region
The invention relates to the technical field of immunology and molecular virology, and particularly discloses a monoclonal antibody of novel coronavirus and a mutant thereof and application of the monoclonal antibody. Complementary determining regions CDR1, CDR2 and CDR3 of a heavy chain variable region of the monoclonal antibody or an antigen binding fragment thereof respectively have amino acid sequences as shown in SEQ ID NO: 1, SEQ ID NO: 2 and SEQ ID NO: 3; and complementary determining regions CDR1, CDR2 and CDR3 of a light chain variable region of the monoclonal antibody or the antigen binding fragment thereof respectively have amino acid sequences as shown in SEQ ID NO: 4, SEQ ID NO: 5 and SEQ ID NO: 6. The monoclonal antibody disclosed by the invention can be combined with the S protein RBD of the novel coronavirus and various mutant strains thereof with high affinity, is strong in neutralizing activity, and has ideal clinical application value for preventing and treating infection of the novel coronavirus and various mutant strains thereof.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Hybridoma cell strain secreting high-neutralization activity infectious bursal disease virus (IBDV) monoclonal antibody
ActiveCN103232974AHigh neutralization potencyAnd high potencyMicroorganism based processesImmunoglobulins against virusesMonoclonalInfectious bursitis
The invention relates to a hybridoma cell strain secreting a high-neutralization activity infectious bursal disease virus (IBDV) monoclonal antibody, and belongs to the field of biotechnology. The hybridoma cell strain C128 is screened from an established bank of hybridoma cells secreting the IBDV monoclonal antibody, and a mice ascites monoclonal antibody prepared by the hybridoma cell strain has IBDV neutralization titer of 1010. The high-neutralization activity IBDV monoclonal antibody secreted by the hybridoma cell strain C128 has high neutralization titer and a wide IBDV strain resistance range, can be used for clinical treatment on IBD-infected animals, and has obvious curative effects, good safety and no adverse side reaction. A monoclonal antibody preparation prepared by the hybridoma cell strain C128 keeps neutralization titer after being preserved for 2 years and has good stability.
Owner:JIANGSU ACAD OF AGRI SCI
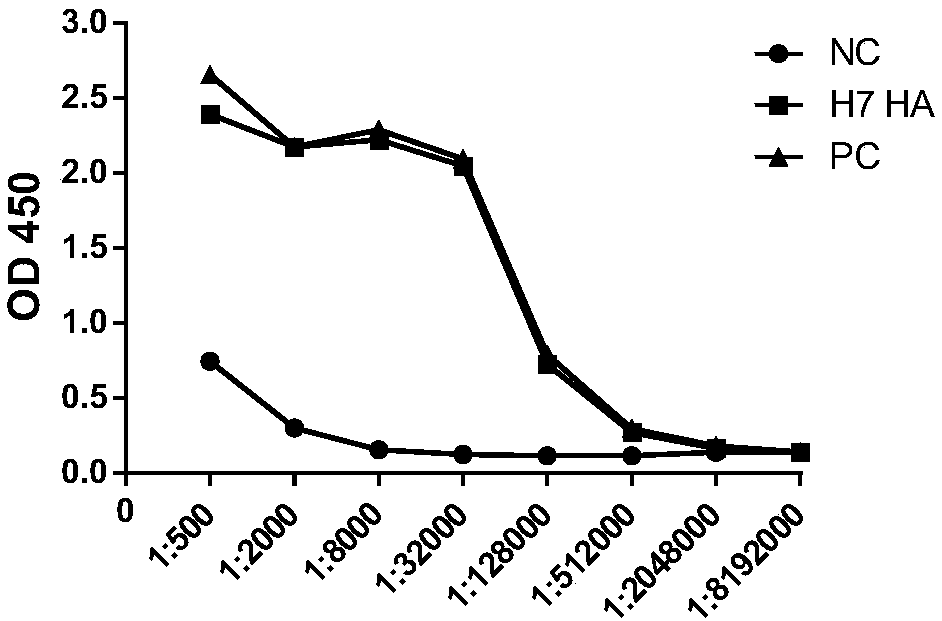
Neutralizing monoclonal antibodies for resisting H7N9 influenza virus
InactiveCN108794624AHigh neutralizing activityEasy to express and purifyImmunoglobulins against virusesAntiviralsMonoclonal antibodyVirus antigen
The invention discloses neutralizing monoclonal antibodies for resisting the H7N9 influenza virus and particularly discloses two monoclonal antibodies aiming at the H7N9 influenza virus. The antibodies has evident combining activity to H7N9 influenza virus antigens.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Efficient-secretion canine parvovirus resistant monoclonal antibody hybridoma cell A135 strain
InactiveCN104877968AImprove biological activityHigh neutralization potencyImmunoglobulins against virusesAntiviralsBALB/cDisease
The invention relates to an efficient-secretion canine parvovirus (CPV) resistant monoclonal antibody hybridoma cell A135 strain and belongs to the technical field of biology. The hybridoma cell A135 strain selected from the secretion CPV resistant monoclonal antibody hybridoma cell bank is excellent in biological performance and is injected with ascetic fluid generated in a BALB / C mouse peritoneal, so that neutralizing titer is up to 1010. In addition, the hybridoma cell A135 strain has moderate neutralizing capacity for various CPV subtype virus strain such as CPV-2a, CPV-2b, CPV-2c (a) and CPV-2c (b) and CPV strains from foxes and raccoon dogs, is wide in anti-CPV strain range and is used for clinical treatment of attacked dogs with CPV diseases, and effective rate is up to 100%.
Owner:JIANGSU ACAD OF AGRI SCI
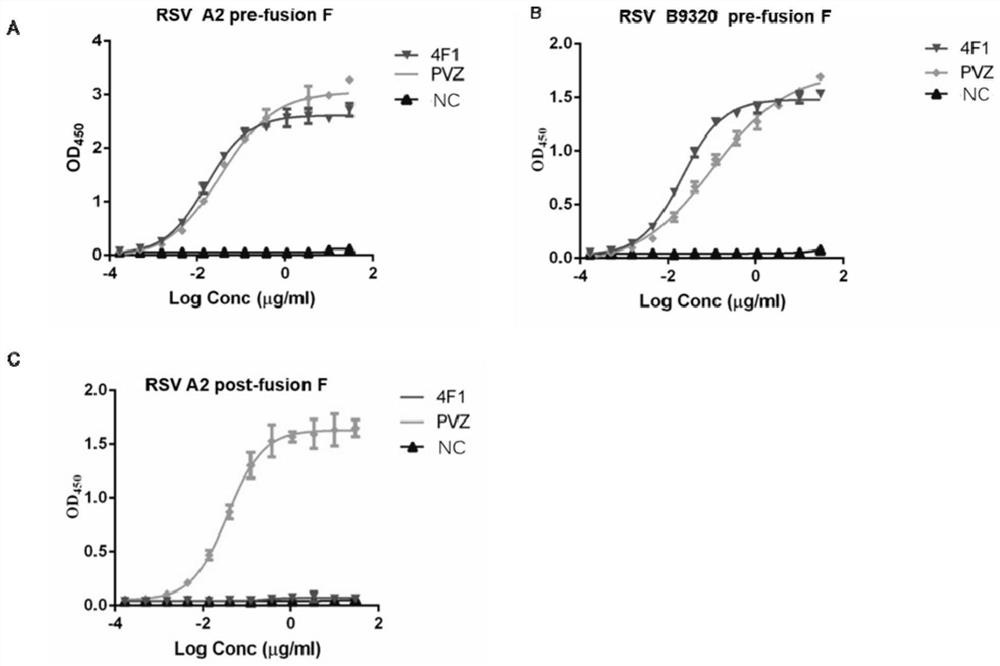
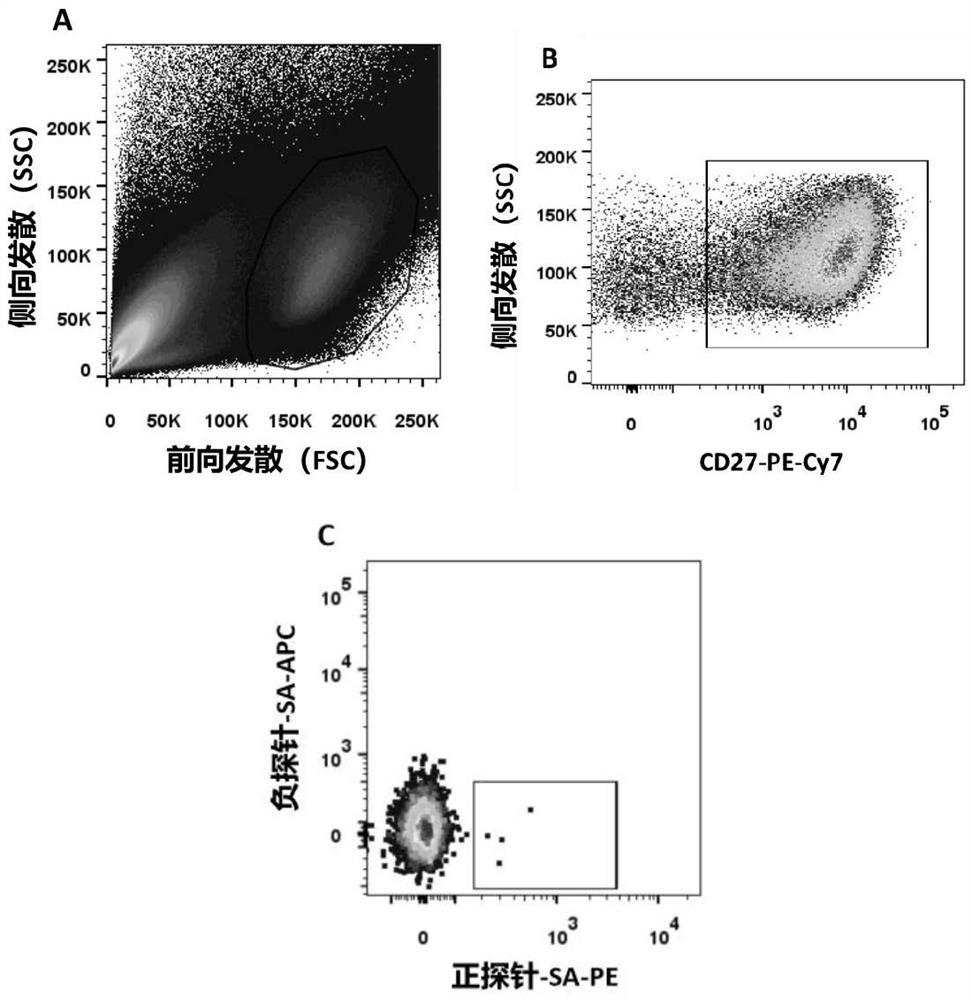
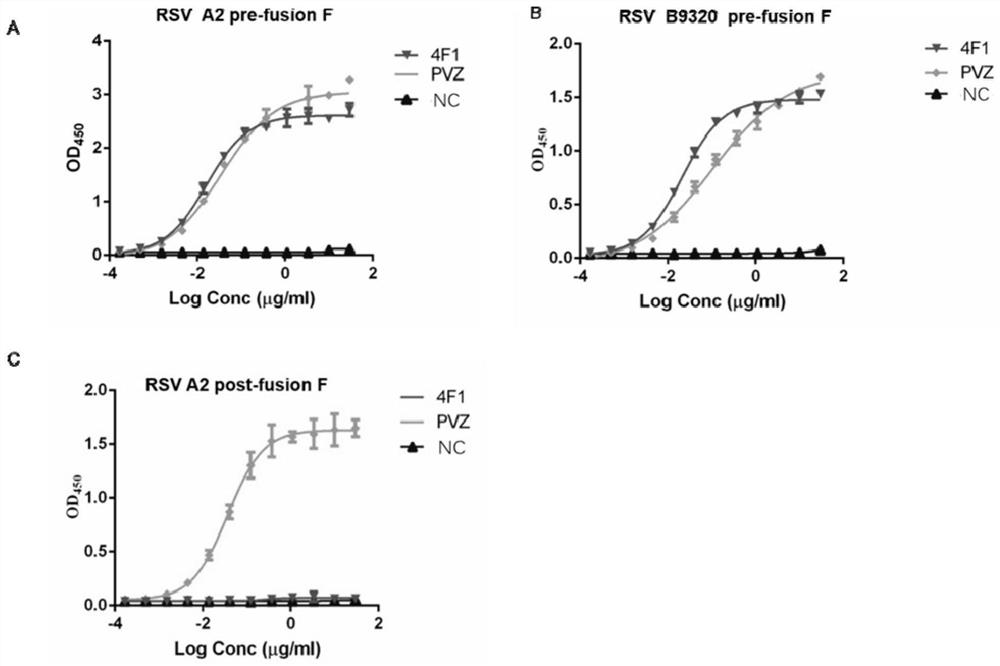
Anti-respiratory syncytial virus fully human broad-spectrum neutralizing antibody 4F1 and application thereof
ActiveCN111606993AHigh neutralizing activityInhibit or prevent infestationImmunoglobulins against virusesAntiviralsAntibody fragmentsF protein
The invention discloses a fully-human neutralizing antibody of anti-respiratory syncytial virus fusion protein and application of the fully-human neutralizing antibody. Specifically, the invention discloses a fully human monoclonal antibody 4F1 aiming at respiratory syncytial virus fusion protein (F protein) and pre-fusion F protein (preF protein), a nucleic acid sequence for encoding an antibodyand an antibody fragment, and a preparation method of the nucleic acid sequence. In-vitro and in-vivo experiments prove that the 4F1 antibody can effectively prevent and control RSV infection, has lowimmunogenicity for a human body, can avoid antibody-mediated immunological rejection of human anti-mouse and other species sources, and can be clinically used for preventing and treating respiratorysyncytial virus infection.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI +1
Fully human monoclonal antibody for resisting novel coronavirus and application of fully human monoclonal antibody
ActiveCN112794898AHigh affinityHigh neutralizing activityImmunoglobulins against virusesAntiviralsMutationIge binding epitopes
The invention relates to the field of biological medicine, and discloses a fully human monoclonal antibody for resisting novel coronavirus and application of the fully human monoclonal antibody. An SARS-CoV2 receptor binding domain (RBD) is used as a probe, flow cytometry is used for screening B lymphocytes of a new coronary pneumonia rehabilitation patient, and the affinity constant of the prepared monoclonal antibody and the RBD is 4.03 nM. X-ray crystal diffraction is used for analyzing the three-dimensional structure of the antibody and RBD compound to find that binding epitopes of the antibody and a host cell receptor (ACE2) are overlapped, binding of the RBD and the ACE2 can be competitively blocked, the virus is prevented from infecting host cells, some genetic mutations of the new coronavirus can be coped with. The median effective concentration (IC50) for blocking pseudoviruses (accumulated B.1. 1.7 mutant strain genes and D614G) from infecting host cells is 0.12 ng / mL, and the application prospect of diagnosing, treating and preventing the new coronal pneumonia is achieved.
Owner:SUZHOU YUZHIBO BIOLOGICAL TECH CO LTD
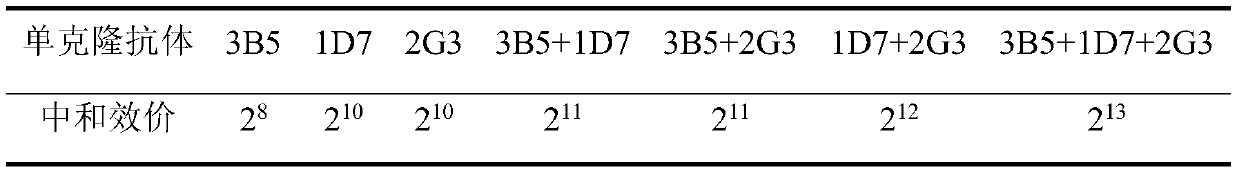
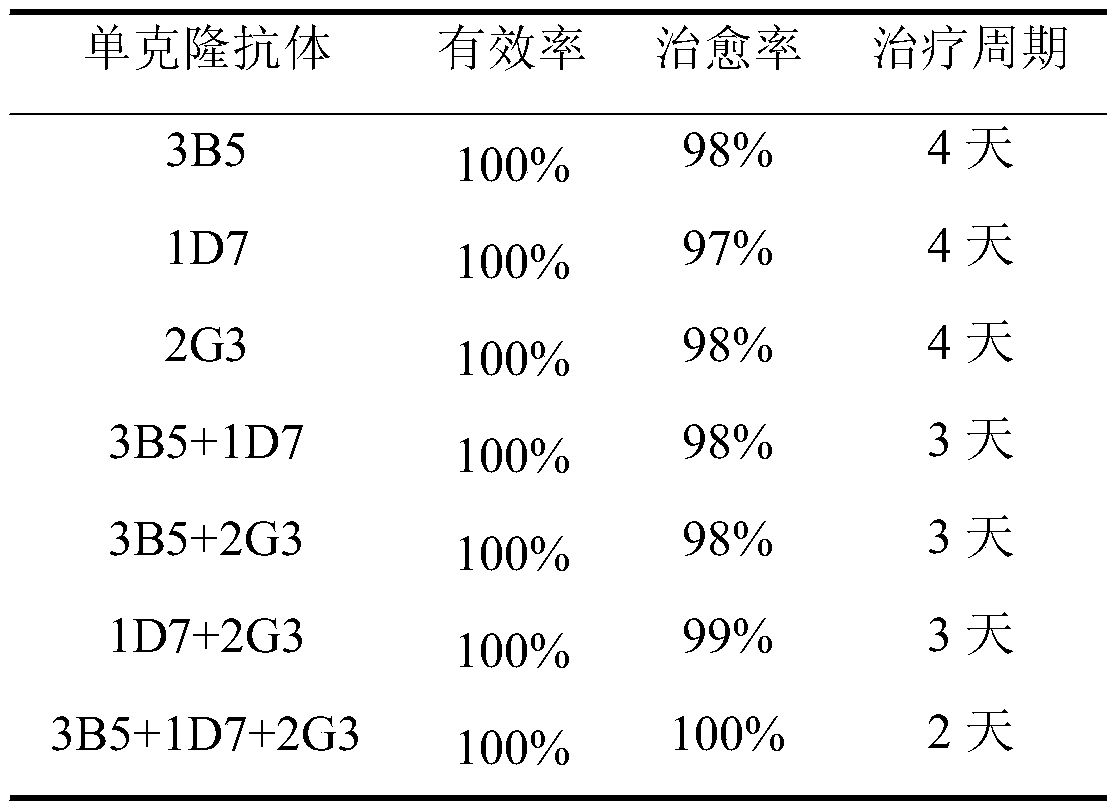
Monoclonal antibody hybridoma cell 3B5 strain capable of secreting H protein for resisting canine distemper virus
ActiveCN110777121AGood biological propertiesStrong ability to neutralize virusesImmunoglobulins against virusesAntiviralsCanine distemper virus CDVTherapeutic effect
The invention discloses a monoclonal antibody hybridoma cell 3B5 strain capable of secreting an H protein for resisting a canine distemper virus, and belongs to the biotechnology field. One hybridomacell strain 3B5 with a high neutralization activity is screened from an established bank of 127 strains of hybridoma cells, and an epitope is amino acids on the 228th-237th site of the H protein for resisting the canine distemper virus. The neutralization valence of an antibody composite therapeutic agent formed by the monoclonal antibody 3B5 and monoclonal antibodies 1D7 and 2G3 is 213, and a virus neutralization capability is improved by 8-32 times than the neutralization valence obtained when 3B5, 1D7 and 2G3 are independently used, the clinic application test effect of a monoclonal antibody composite therapeutic agent is improved to 100% from 98% than an effect obtained when the monoclonal antibodies 1D7 and 2G3 are subjected to combined utilization, and a treatment period is shortened to 2 days from 4 days. The monoclonal antibody hybridoma cell 3B5 strain can be used for the clinic treatment of CD (canine distemper) morbidity animals. By use of the strain disclosed by the invention, production cost is lowered, and a treatment effect is improved.
Owner:JIANGSU ACAD OF AGRI SCI
2019-nCoV S-antigens for generating 2019-nCoV neutralizing antibodies and method for preparing 2019-nCoV S-antigens
InactiveCN113248581AImprove immunityEffective immunitySsRNA viruses positive-senseSerum immunoglobulinsAntigenCell membrane
The invention discloses a 2019-nCoV S-antigens for generating 2019-nCoV neutralizing antibodies and a method for preparing the 2019-nCoV S-antigens. According to the 2019-nCoV S-antigens and the method, the 2019-nCoV S-antigens comprise 2019-nCoV S-antigen trimers combined with a cell membrane based on a Flag tag. The 2019-nCoV S-antigens can generate high-titer neutralizing antibodies so as to more effectively immunize animals.
Owner:江西浩然生物制药有限公司
Novel anti-human NGF antibody
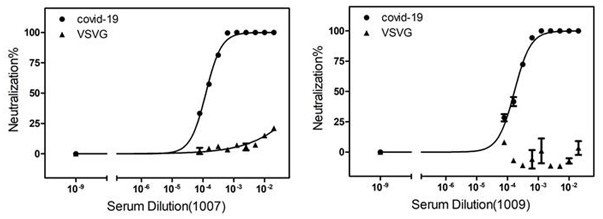
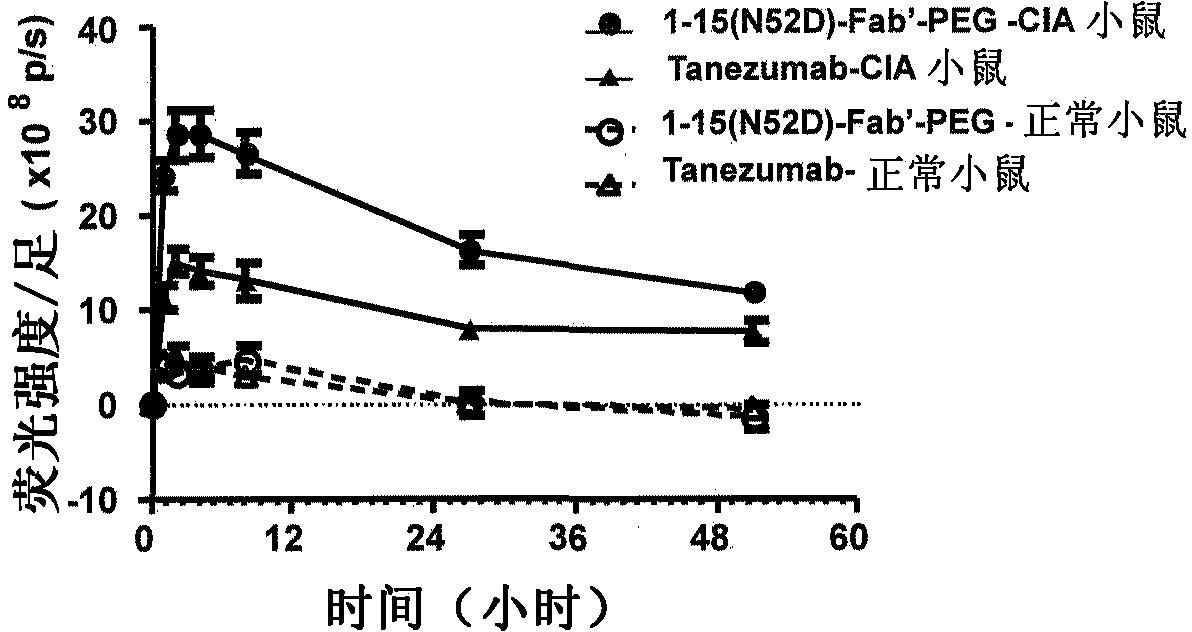
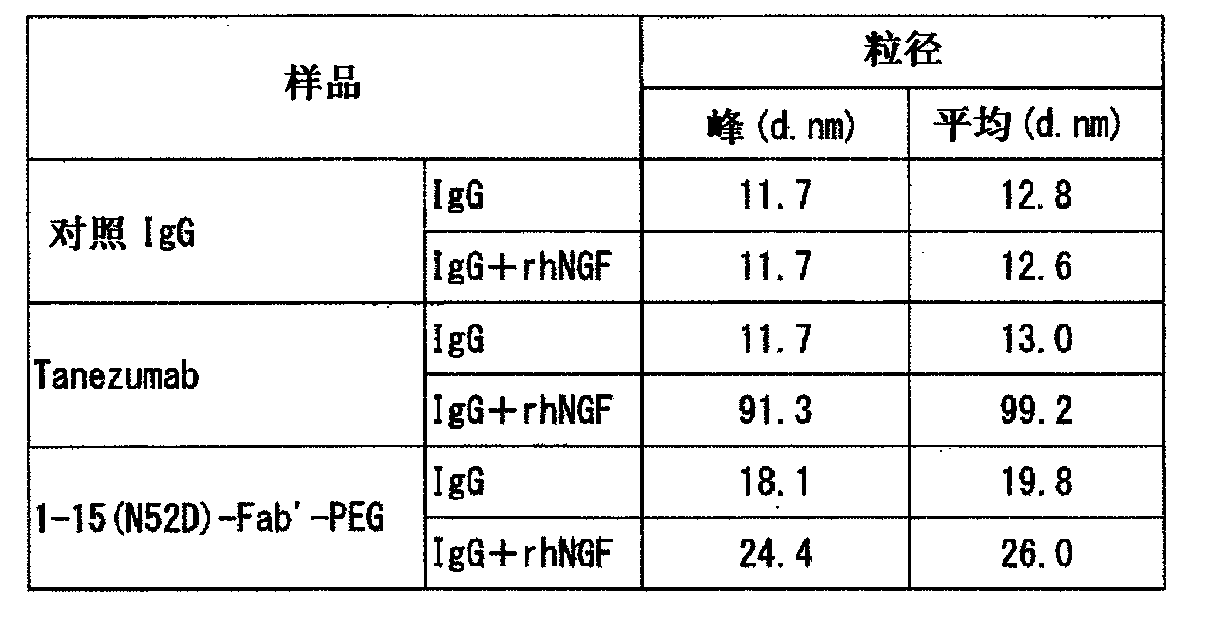
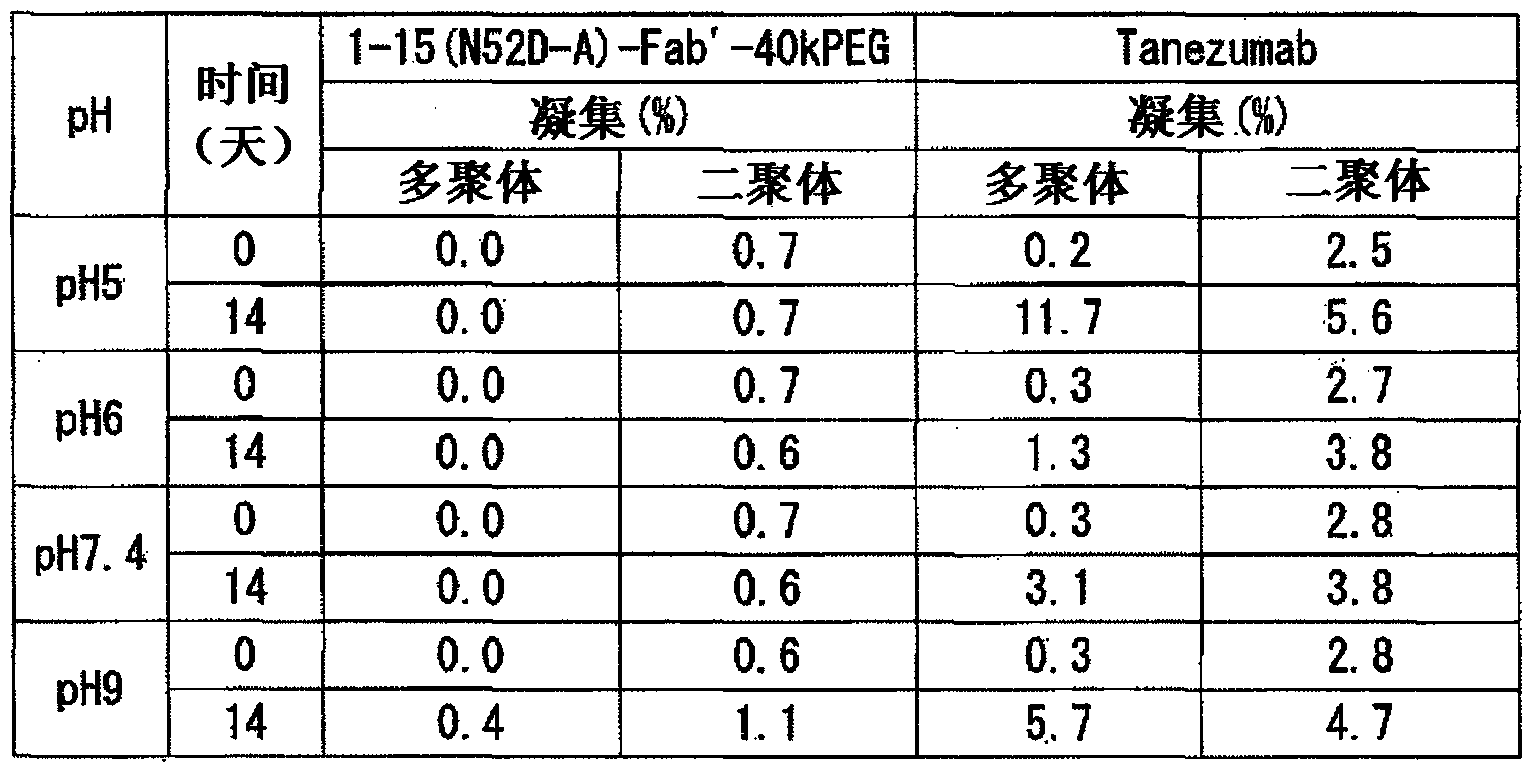
Provided are an anti-human NGF antibody which is reduced in the influence on fetuses and the risk of adverse side effects including thrombosis while keeping a high neutralizing activity, and which has excellent safety, or an antigen-binding fragment thereof; and a means, utilizing the antibody or an antigen-binding fragment thereof , for preventing or treating various diseases for which human NGF is involved in the development of a disease state. An Fab' fragment of an anti-human NGF antibody comprises a heavy-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 6 and a light-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 4.
Owner:ASTELLAS PHARMA INC
Immunoenhancer as well as application thereof in vaccine preparation
ActiveCN111138535APrevent intrusionHigh neutralizing activitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantImmune complex deposition
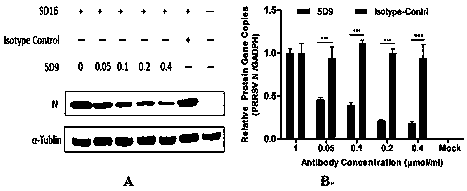
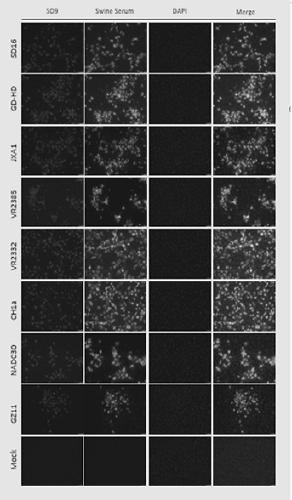
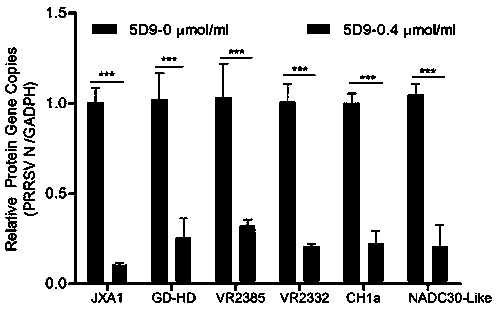
The invention adopts a monoclonal antibody 5D9 with neutralization activity to both PRRSV-I and PRRSV-II viruses to prepare an immunoenhancer. When a formed immune complex is combined with an adjuvantto immunize mice, T cells secreted by IFN-gamma are significantly increased, suggesting that combined immunization of the PRRSV specific antibody 5D9 and the normal oil in water adjuvant can enhanceCTL reaction in the process of inactivating virus immunity. Animal tests show that the immune protection rate of the immune complex prepared by the method can achieve a higher protection effect than that of a vaccine and a commercialized attenuated vaccine prepared only with a commercial adjuvant ISA 206.
Owner:NORTHWEST A & F UNIV
Enterovirus 71 (EV71) monoclonal antibody and application thereof
ActiveCN102409028AHigh school and activeStrong guiding significanceImmunoglobulins against virusesMicroorganism based processesLaboratory culturePassive Treatment
The invention provides an enterovirus 71 (EV71) monoclonal antibody and an application thereof. The monoclonal antibody is derived from an EV71 hybrid tumor cell strain, which has a preservation number of CGMCC (China General Microbiological Culture Collection) No.5330. The monoclonal antibody and related reagents thereof have important significance in promoting the treatment and research of severe cases and death cases caused by EV71 in China, and have extremely important value in further promoting clinical research, epidemic development and passive treatment of EV71.
Owner:SINOVAC BIOTECH
A kind of preparation method of recombinant porcine epidemic diarrhea virus antibody
InactiveCN106146658BNo immune responseStrong targetingImmunoglobulins against virusesFermentationEscherichia coliAntigen
The present invention relates to a preparation method of a recombinant porcine epidemic diarrhea virus antibody. The preparation method of the recombinant porcine epidemic diarrhea virus antibody: select the mode of co-expression of antigen and antibody, and combine PEDV antigen gene S1 and anti-PEDV ScFv gene Co-expressed in the periplasmic cavity of Escherichia coli, three rounds of panning were performed by flow cytometry; the PEDV antibody was purified after expressing the ScFv gene in Escherichia coli, and the specificity of the PEDV ScFv antibody was detected by ELISA method, which was named PEDV‑ScFv; The construction of the anti-PEDV ScFv library and the preparation of the anti-PEDV antibody were carried out in two steps. The pig-derived PEDV antibody provided by the invention does not generate an immune response, is highly targeted, is easier to screen for antibodies with high neutralizing activity, and has a short preparation cycle and high speed.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Anti-SARS-CoV-2 single-chain antibody, and preparation method and application thereof
ActiveCN113698477AHigh affinityEfficient combinationBacteriaMicroorganism based processesSingle-Chain AntibodiesAntiendomysial antibodies
The invention relates to an anti-SARS-CoV-2 single-chain antibody, and a preparation method and application thereof. The complementary determining region of the anti-SARS-CoV-2 single-chain antibody comprises a CDR1 and a CDR3, wherein the CDR1 comprises an amino acid sequence as shown in SEQ ID NO.1 or SEQ ID NO.2, and the CDR3 comprises an amino acid sequence as shown in SEQ ID NO.3 or SEQ ID NO.4. The anti-SARS-CoV-2 single-chain antibody has high affinity, can be efficiently combined with SARS-CoV-2, is unlikely to dissociate, has a high virus neutralizing activity, can be efficiently expressed in a prokaryotic system, has a standard and controllable expression process, can greatly reduce the production cost, is small in molecular weight, is stable in physicochemical properties and good in heat resistance, can greatly reduce the transportation cost as a prevention and treatment drug, and has an important application value in the field of SARS-CoV-2 prevention and treatment.
Owner:厦门福宸百奥生物技术有限公司 +2
Preparation method of recombinant porcine epidemic diarrhea virus antibody
InactiveCN106146658ANo immune responseStrong targetingImmunoglobulins against virusesFermentationEscherichia coliAntigen
The invention relates to a preparation method of a recombinant porcine epidemic diarrhea virus antibody, comprising: selecting an antigen antibody co-expression mode, co-expressing PEDV (porcine epidemic diarrhea virus) antigen gene and anti-PEDV ScFv gene in Escherichia coli periplasmic cavity, and performing triple sorting by using flow cytometry; using Escherichia coli to express the ScFv gene, then purifying the PEDV antibody, detecting specificity of the PEDV ScFv antibody by using ELISA (enzyme-linked immuno sorbent assay), and naming the antibody as PEDV-ScFv; wherein two specific steps are performed: constructing an anti-PEDV ScFv library and preparing the anti-PEDV antibody. The porcine-derived PEDV antibody provided herein never causes immune response, has high specificity, enables easier screening of an antibody with high neutralizing activity, and has short preparation cycle and high preparation speed.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Monoclonal antibody against human midkine
ActiveUS20150203573A1Good reactivityHigh neutralizing activityAnimal cellsSugar derivativesWilms' tumorAmino acid
A monoclonal antibody, which recognizes at least two amino acids among amino acids located at position 69, position 79, position 81 and position 102 of human midkine, has been found to have excellent reactivity with and excellent neutralizing activity against human midkine. Moreover, the activity of suppressing the proliferation of tumor has been observed in the antibody having excellent neutralizing activity. The use of the antibody of the present invention makes it possible to treat cancer effectively and to detect or purify midkine efficiently.
Owner:NAGOYA UNIVERSITY +1
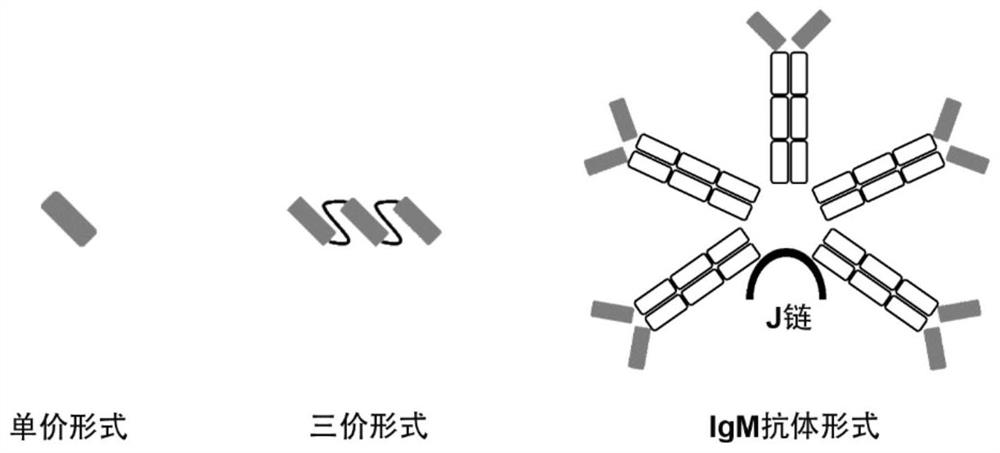
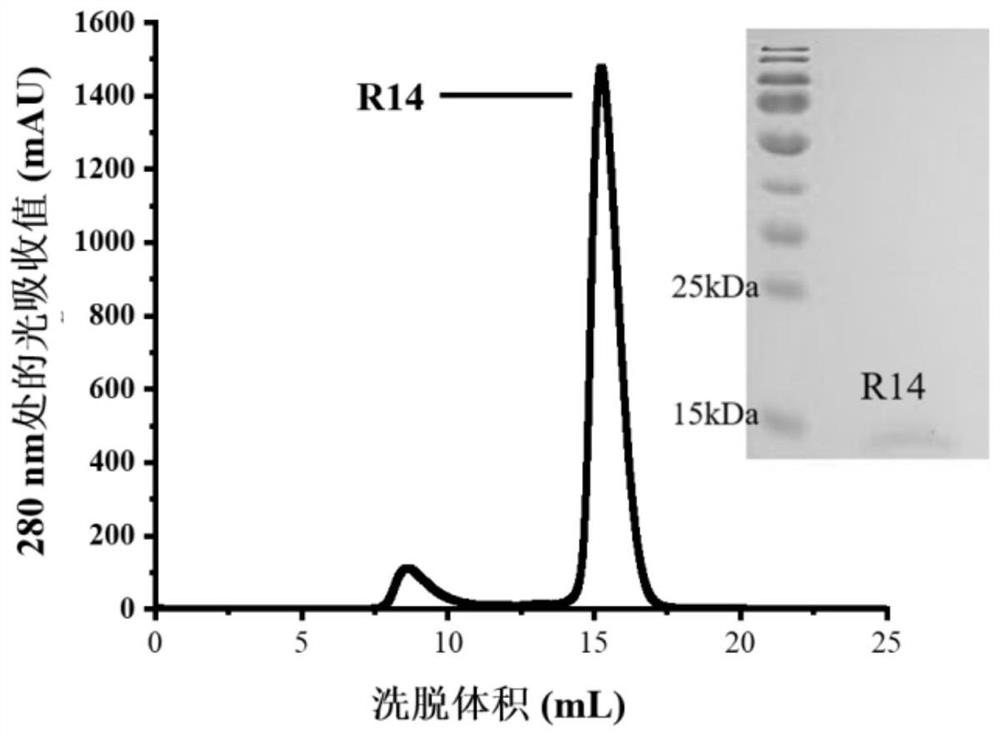
Construction body of nano antibody R14 and application thereof
ActiveCN114805560AHigh neutralizing activityAchieve immunityImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesVariant strain
The invention provides a construction body (including a multivalent nano antibody and a nano antibody fusion protein) of a nano antibody R14 based on specific binding SARS-CoV-2RBD, a related product thereof and an application of the construction body. The construction body (including multivalent nano-antibody and nano-antibody fusion protein) of the nano-antibody R14 based on the specific binding SARS-CoV-2RBD can effectively inhibit SARS-CoV-2 infection and variant strain infection thereof, can be dosed by atomization, can directly reach the lung, takes effect quickly, is long in half-life period, and can be used for preparing the nano-antibody R14 of the SARS-CoV-2RBD. And a more effective treatment strategy is provided for clinically preventing or treating infection of the new coronavirus and the variant thereof.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Neutralizing antibody against Junin virus, preparation method thereof and application thereof
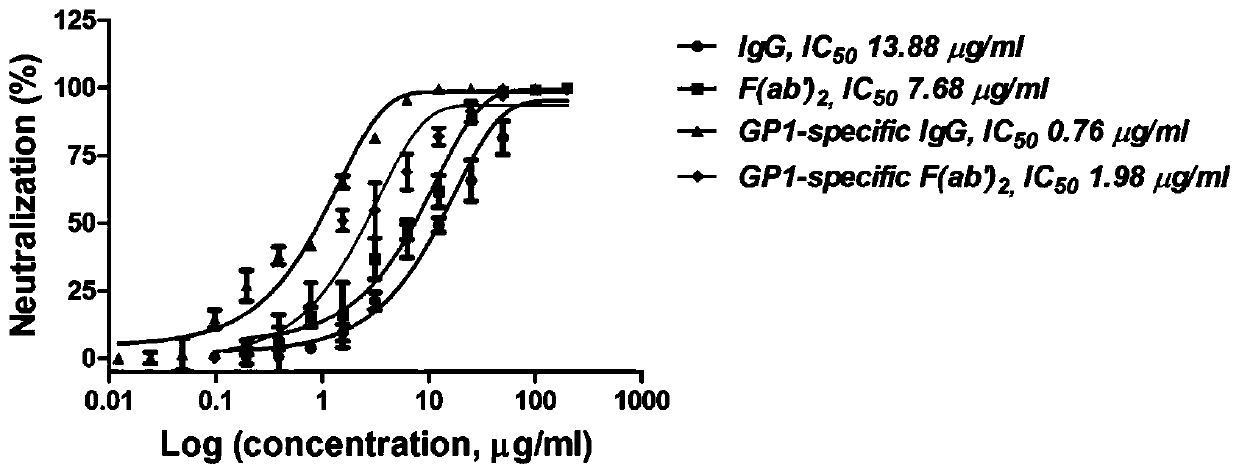
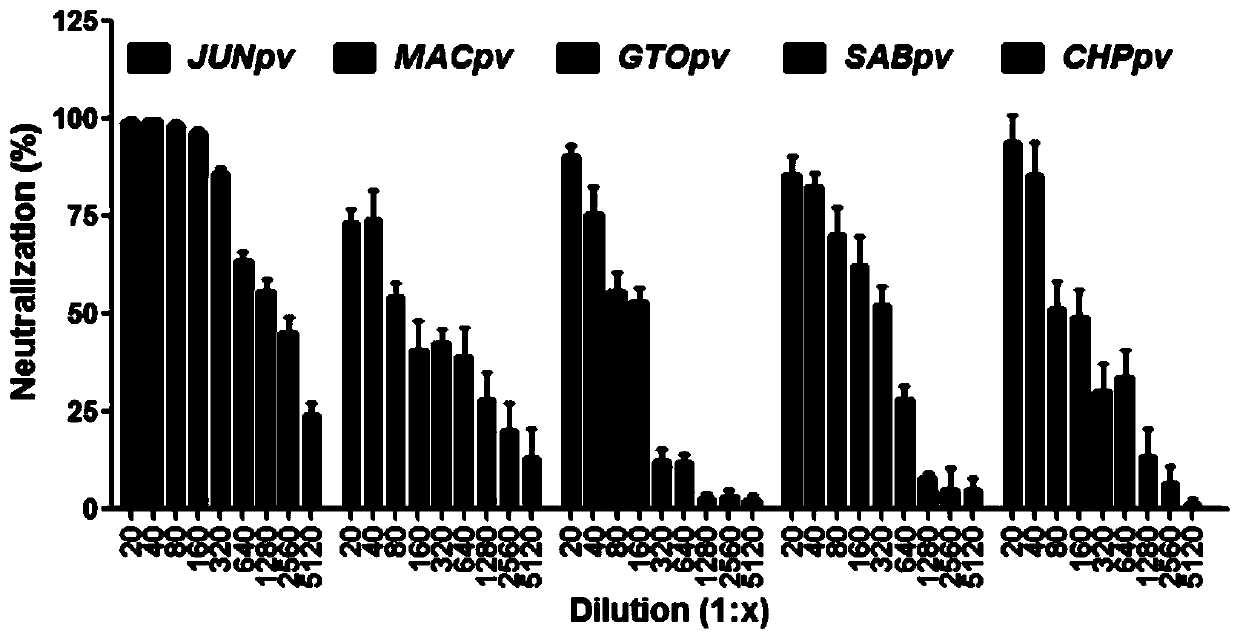
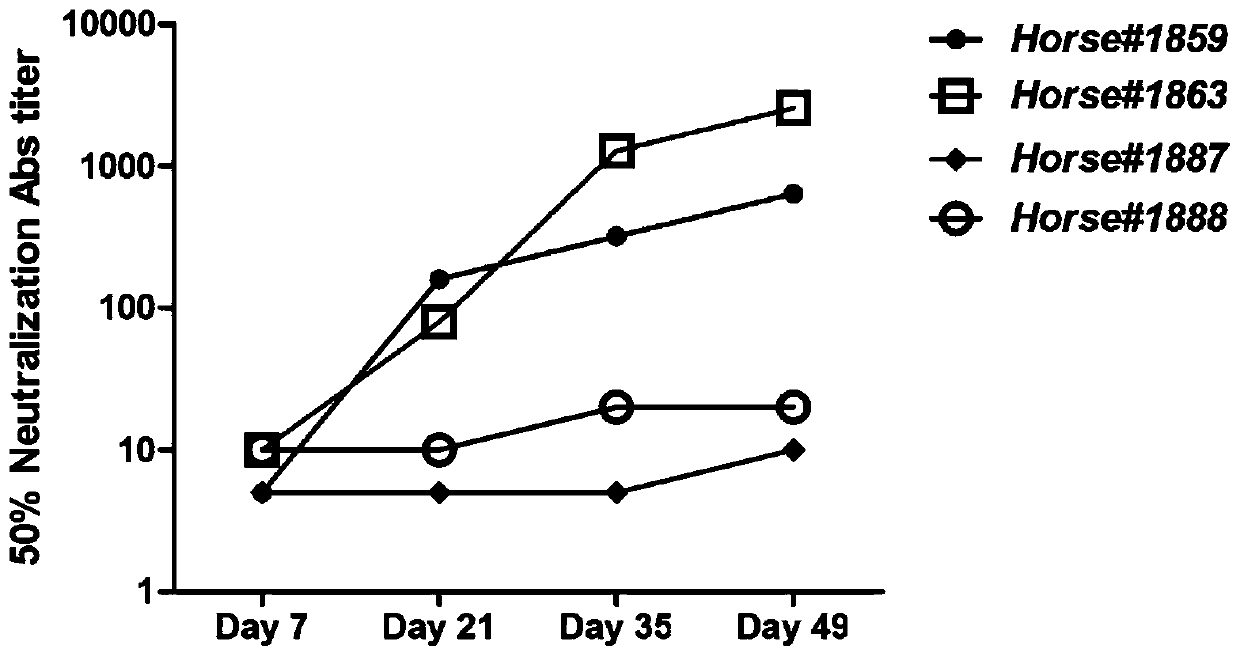
ActiveCN110204613AHigh neutralizing activitySsRNA viruses negative-senseImmunoglobulins against virusesSerum igeEmergency treatment
The invention provides a neutralizing antibody against Junin virus, a preparation method thereof and an application thereof, which relate to the technical field of bio-medicine. The method comprises the following steps: immunizing an animal with a prepared Junin virus antigen to obtain a polyclonal antibody, then performing specific purification of the Junin virus envelope glycoprotein subunit 1 on the polyclonal antibody, preparing the antibodies with high neutralizing activity, and obtaining a large amount of horse antiserum products for clinical treatment of Argentine hemorrhagic fever. Thepreparation method and the product prepared therefrom contribute to the emergency treatment of Argentine hemorrhagic fever.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Adjuvant of novel coronavirus vaccine, application of adjuvant and novel coronavirus bivalent recombinant vaccine
ActiveCN113842455ABroad-spectrumSynergisticSsRNA viruses positive-senseViral antigen ingredientsAntigenCell immunity
The invention discloses an adjuvant of a novel coronavirus COVID-19 vaccine and a developed bivalent vaccine which contains a novel coronavirus epidemic HuB strain and a South African mutant strain B.1.351 antigen. The adjuvant disclosed by the invention has the advantages of being more suitable for a stable sodium acetate buffer solution system of a COVID-19 vaccine protein, and high in neutralizing antibody and cellular immune level. On the basis, the bivalent COVID-19 vaccine invented by the invention can have a good protection effect on infection of the novel coronaviruses, especially the HuB strain, the South African mutant strain and a Delta mutant strain.
Owner:BEIJING HEALTH GUARD BIOTECH
Neutralizing monoclonal antibody capable of resisting enterovirus D68 in broad-spectrum way
ActiveCN111153988AHigh neutralizing activityEasy to express and purifyImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesMonoclonal
The invention discloses a neutralizing monoclonal antibody capable of resisting an enterovirus D68 in a broad-spectrum way. Particularly, the invention discloses two strains of monoclonal antibodies which aim at the enterovirus D68. The monoclonal antibodies have obvious binding and neutralizing activity for the enterovirus D68.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Double-antibody composition and application to preparation of COVID-19 (Coronavirus Disease 2019) treatment drugs
ActiveCN112076316AHigh neutralizing activityImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesReceptor
The invention relates to an antibody composition containing a monoclonal antibody specifically bound to an S protein receptor binding domain of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) and a monoclonal antibody specifically bound to an S protein N-terminal structural domain of the SARS-CoV-2. Compared to the monoclonal antibodies separately used, the antibody composition is significantly improved in the aspect of the neutralizing activity on novel coronavirus infected cells, the neutralizing activity is respectively increased to 6.4 times of that of an anti-S protein receptor binding domain and 3.8 times of that of an anti-S protein N-terminal structural domain, and the antibody composition has a remarkable synergistic effect. The invention further relates to application of the antibody composition to preparation of COVID-19 treatment and / or prevention drugs. The antibody composition can be prepared on a large scale by stable engineered strains and an industrial pharmaceutical method, and has a huge industrial prospect.
Owner:ACADEMY OF MILITARY MEDICAL SCI
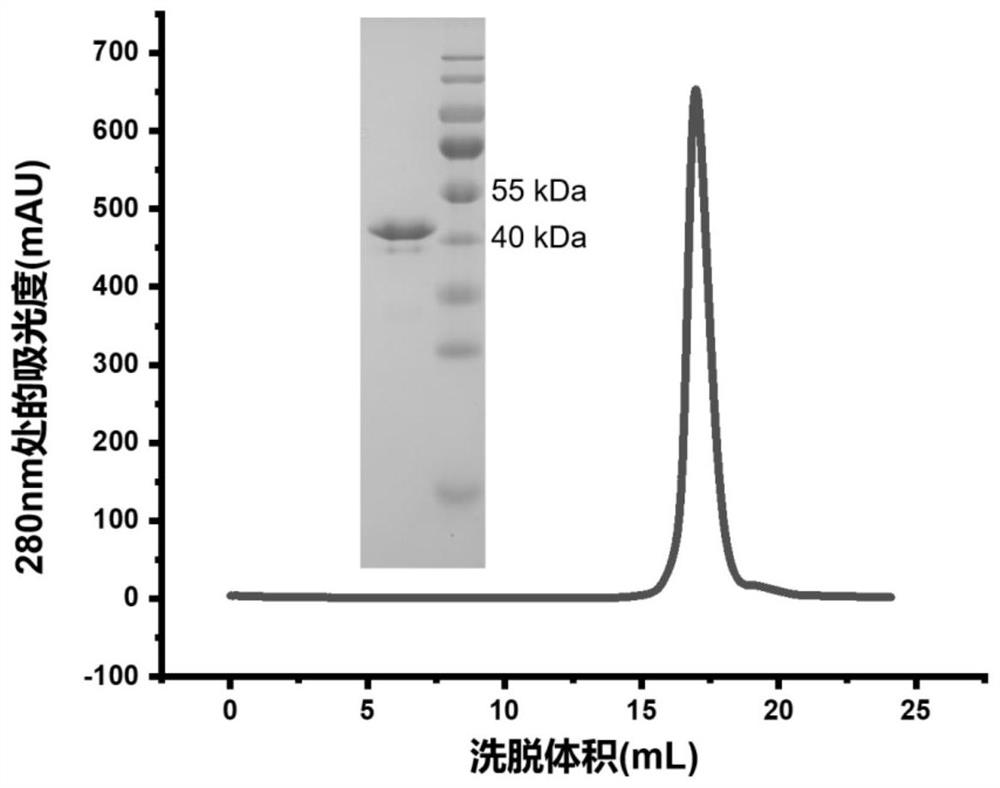
Construction body of nano antibody S43 and application thereof
ActiveCN114763380AHigh neutralizing activityAchieve immunityAntibody mimetics/scaffoldsImmunoglobulins against virusesVariant strainAntibody
The invention provides a construction body (including a multivalent nano antibody and nano antibody fusion protein) of a nano antibody S43 based on specific binding of SARS-CoV-2RBD, and a related product and application thereof. The construction body (including multivalent nano-antibody and nano-antibody fusion protein) of the nano-antibody S43 based on specific binding of SARS-CoV-2RBD can effectively inhibit SARS-CoV-2 infection and variant strain infection thereof, can be dosed in an atomized manner, can directly reach the lung, takes effect quickly, is long in half-life period, and can be used for preparing the nano-antibody S43 with the specific binding of SARS-CoV-2RBD. And a more effective treatment strategy is provided for clinically preventing or treating infection of the new coronavirus and the variant thereof.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
A kind of anti-Nipah virus envelope glycoprotein monoclonal antibody and its application
ActiveCN110028579BCombined with effective inhibitionInhibit bindingImmunoglobulins against virusesAntiviralsDiseaseHendra Virus
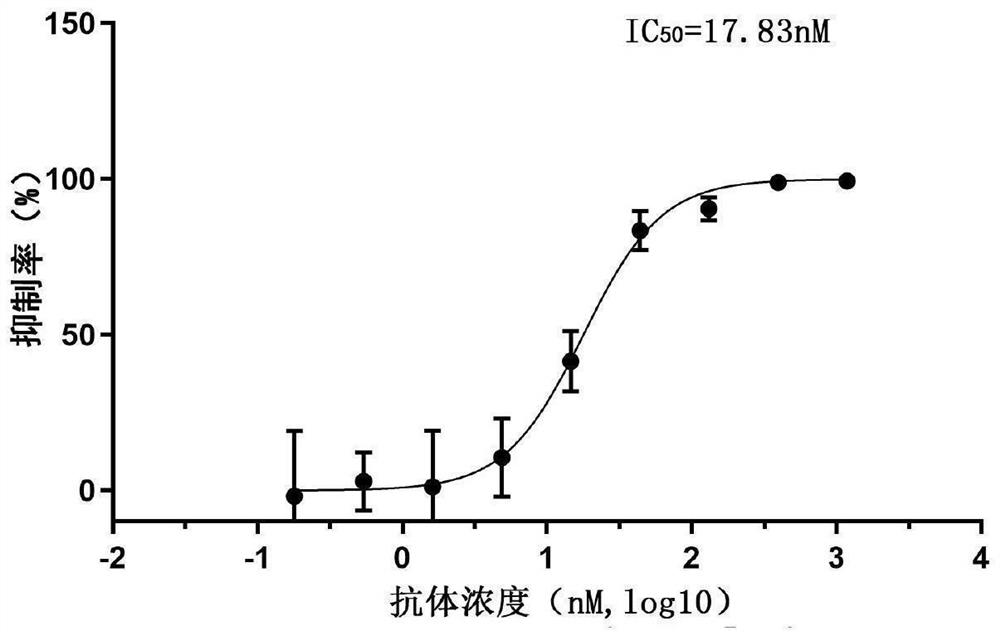
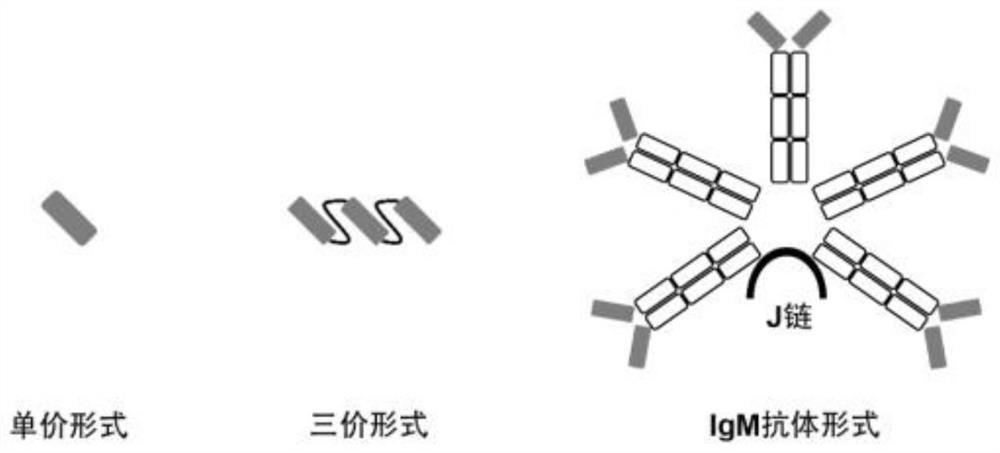
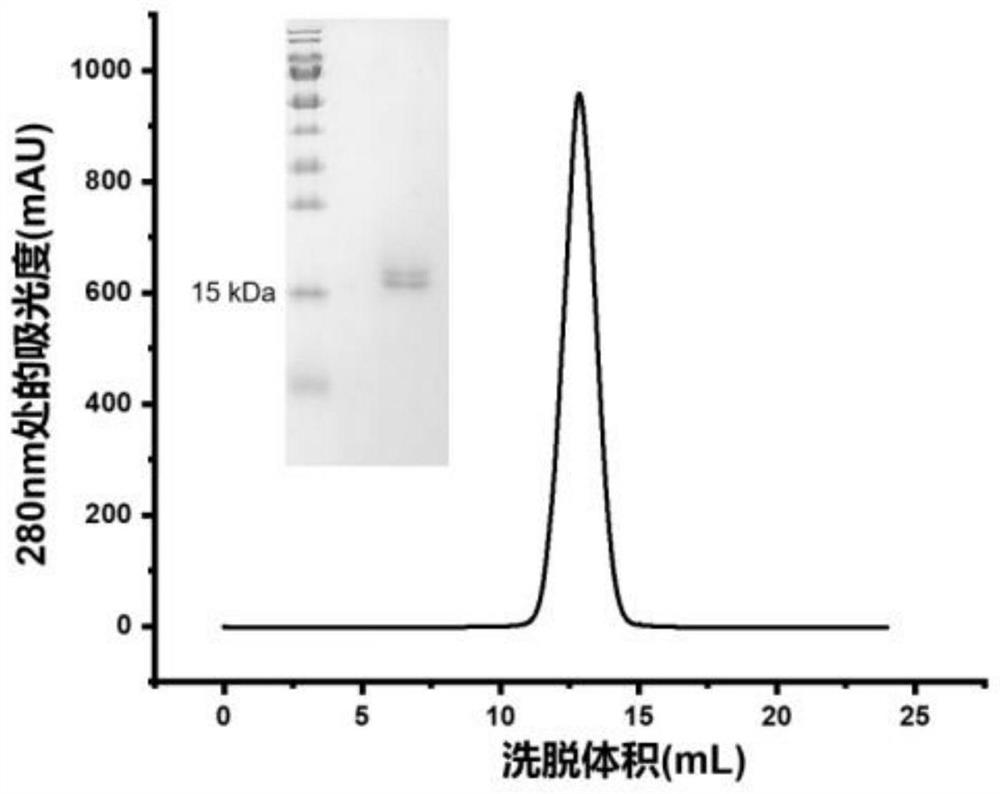
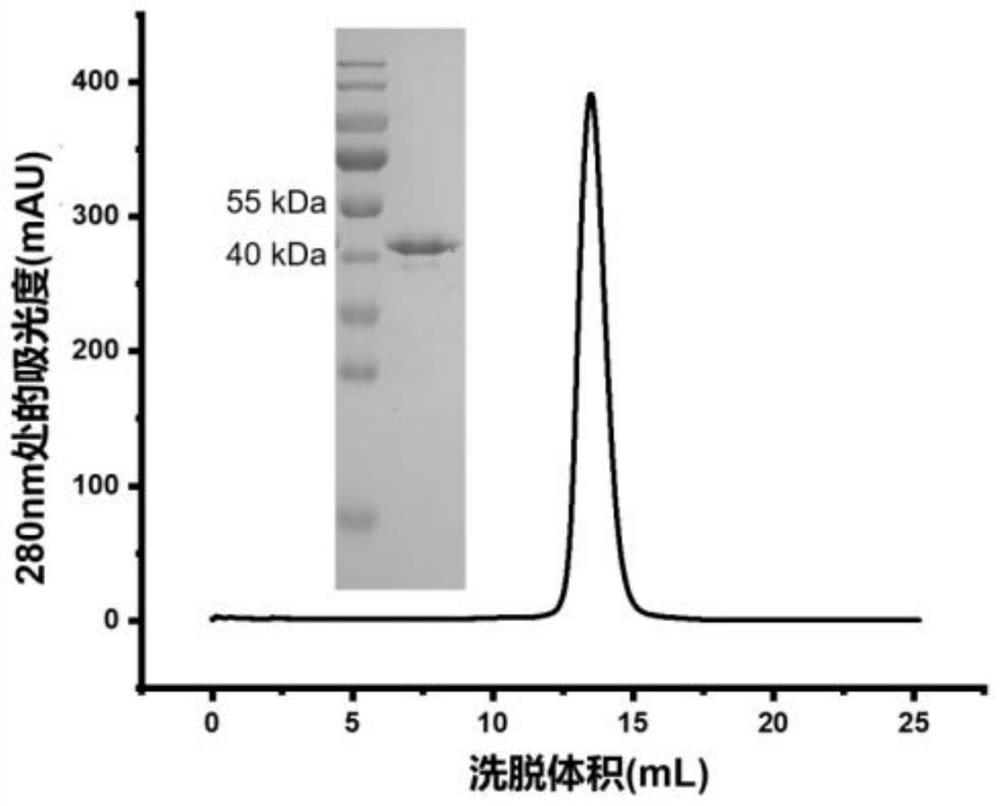
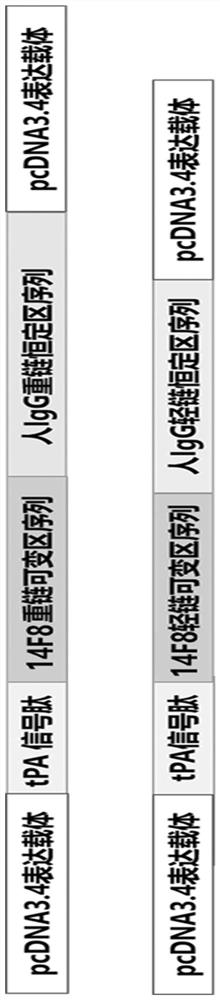
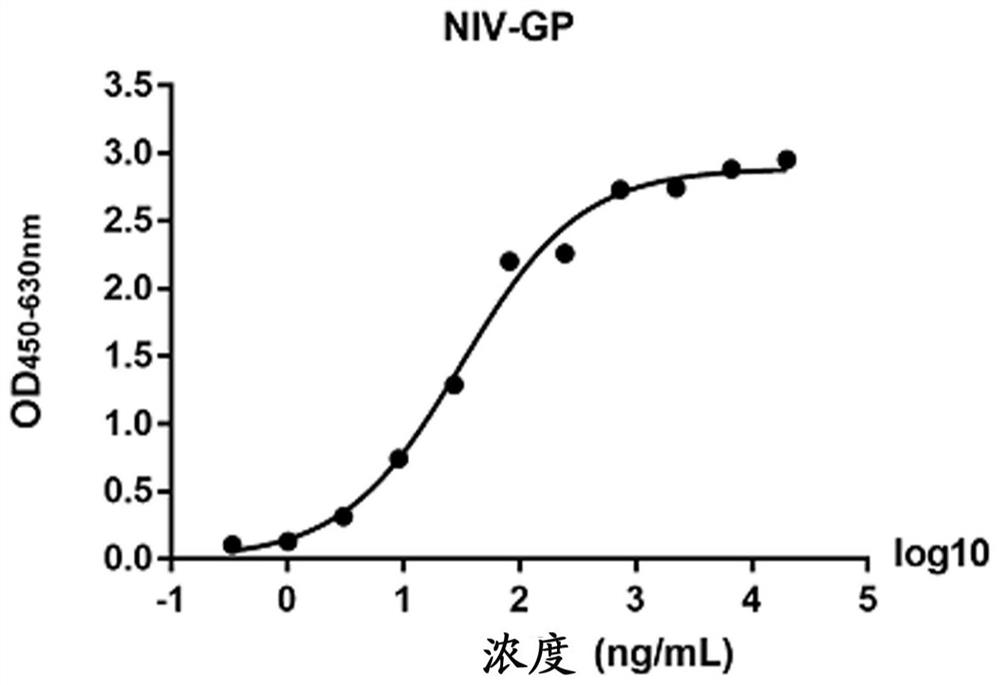
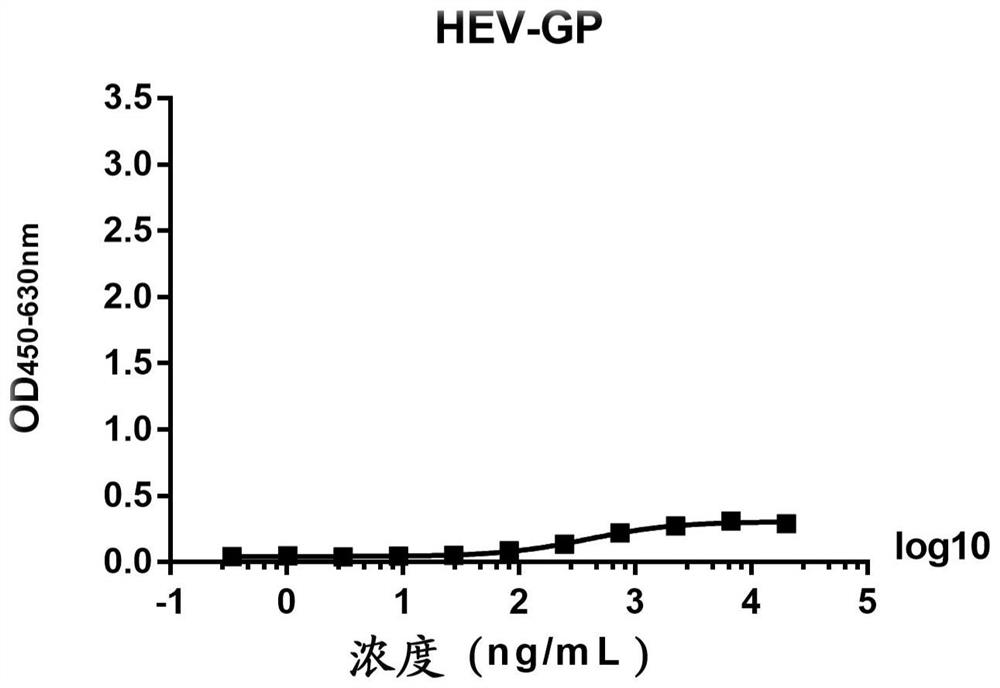
The present invention discloses a monoclonal antibody 14F8 against Nipah virus envelope glycoprotein. The antibody has a unique CDR region and has a binding titer of 0.47 ng / mL with the Nipah virus envelope glycoprotein, and when an antibody concentration is greater than 9.14 ng / mL, an ELISA OD value is greater than 2.0, indicating excellent antigen binding activity. The antibody has a binding titer of 129.66 ng / mL with Hendra virus envelope glycoprotein, and when the antibody concentration is 20,000 ng / mL, the OD value is only 0.29, indicating the 14F8 can be used for detection of the Nipah virus envelope glycoprotein and can effectively distinguish the Nipah virus envelope glycoprotein and the Hendra virus envelope glycoprotein. The present invention also discloses an application of the14F8 monoclonal antibody in preparation of drugs for treating Nipah virus diseases, the antibody can effectively inhibit binding of the Nipah virus envelope glycoprotein to a cellular receptor EFNB2,an IC50 value is 50 ng / mL, besides, neutralizing activity is enhanced with increase of the antibody concentration, and when the antibody concentration exceeds 1 [mu]g / ml, an inhibition rate tends to reach 100%, indicating a prospect of the 14F8 monoclonal antibody as a candidate therapeutic antibody for the Nipah virus diseases.
Owner:ACADEMY OF MILITARY MEDICAL SCI
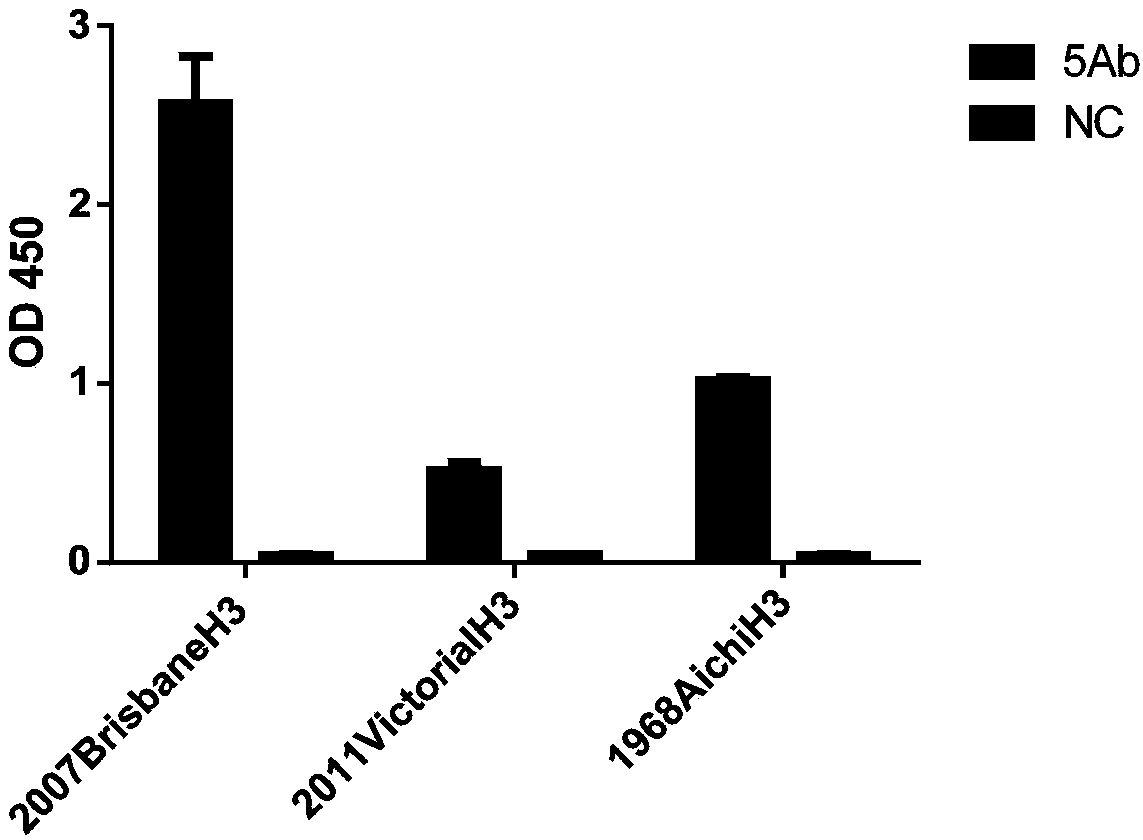
Whole-humanized neutralizing antibody resisting H3N2 influenza virus and application of whole-human neutralizing antibody
InactiveCN111116741AHigh neutralizing activityInhibit or prevent infestationImmunoglobulin superfamilyAntibody mimetics/scaffoldsHemagglutininAntiendomysial antibodies
The invention discloses a whole-humanized neutralizing antibody resisting an H3N2 influenza virus and application of the hole-human neutralizing antibody. Particularly, the invention discloses a whole-humanized monoclonal antibody for the H3N2 influenza virus. The antibody can bind influenza virus hemagglutinin (HA) with a native conformation, can prevent the H3N2 subtype influenza virus from infecting susceptible cells, has lower immunogenicity for human bodies, can avoid medicated immune rejection response of antibodies derived from other specifies such as a human anti-mouse antibody, and has potential values of preventing and treating H3N2 subtype influenza virus infection clinically.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Anti-respiratory syncytial virus fully human broad-spectrum neutralizing antibody 4f1 and its application
ActiveCN111606993BHigh neutralizing activityInhibit or prevent infestationImmunoglobulins against virusesAntiviralsAntibody fragmentsRSV Infections
The invention discloses a fully human neutralizing antibody against fusion protein of respiratory syncytial virus and application thereof. Specifically, the present invention discloses a fully human monoclonal antibody 4F1 against the fusion protein (F protein) and pre-fusion protein (preF protein) of respiratory syncytial virus, nucleic acid sequences encoding the antibody and antibody fragments, and a preparation method thereof. In vitro and in vivo experiments have confirmed that the 4F1 antibody can effectively prevent and control RSV infection. It has low immunogenicity for the human body and can avoid human-anti-mouse and other antibody-mediated immune rejection reactions. It can be used clinically For the prevention and treatment of respiratory syncytial virus infection.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI +1
Human neutralizing antibody or antigen binding fragment thereof and application thereof
ActiveCN114573691AHigh school and activeGood neutralizing activityImmunoglobulins against virusesAntiviralsAntigenHeavy chain
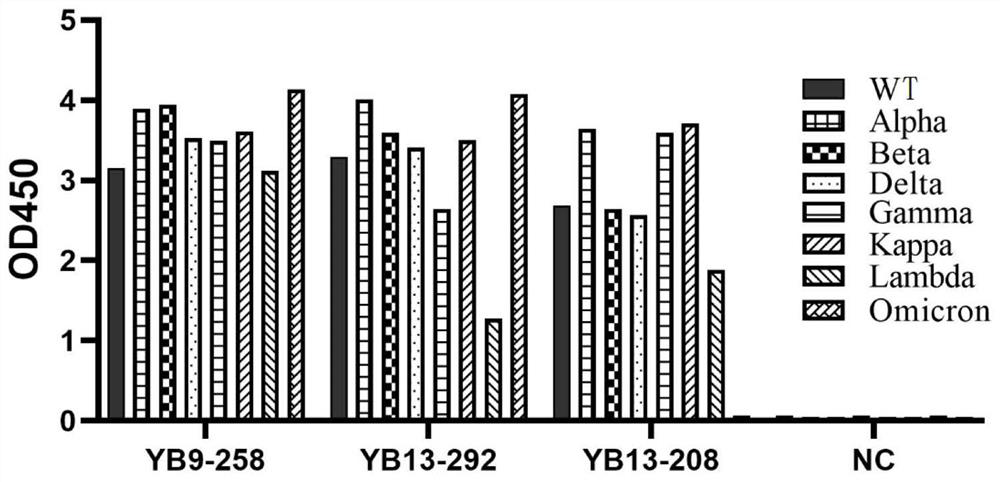
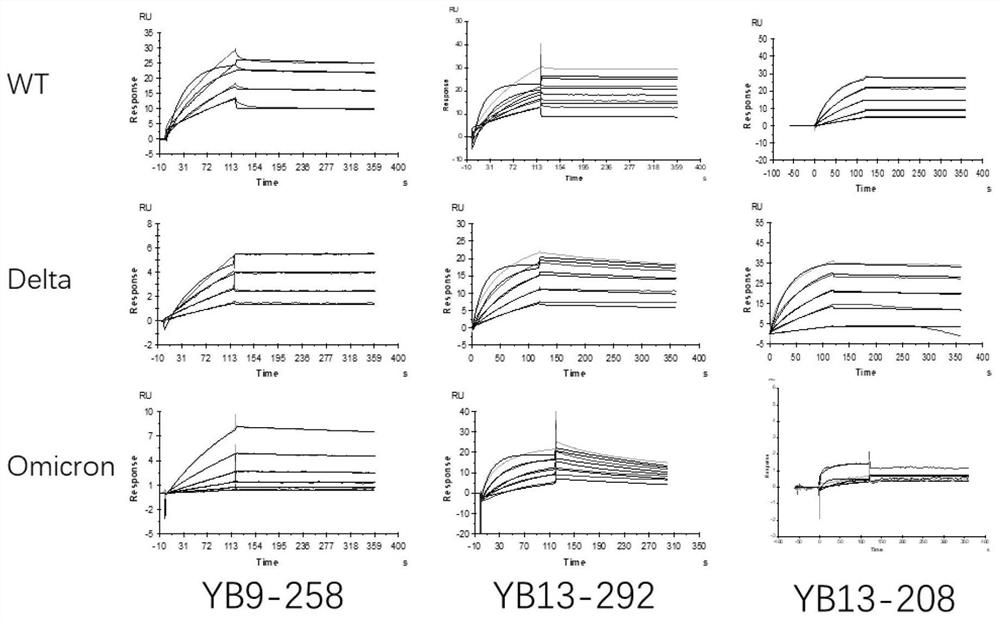
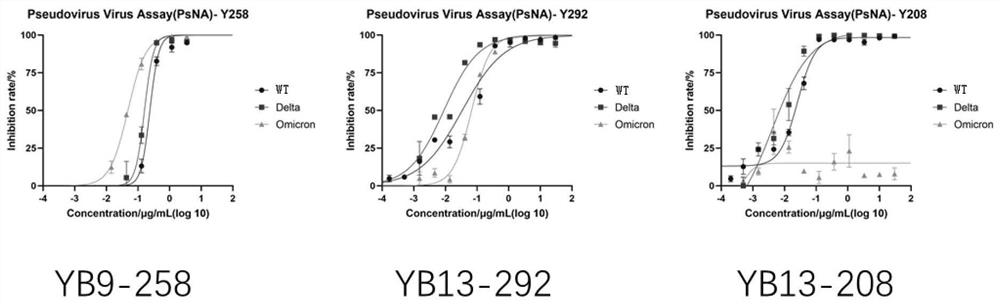
The invention relates to a human neutralizing antibody or an antigen binding fragment thereof and application thereof, the human neutralizing antibody or the antigen binding fragment comprises a heavy chain variable region and / or a light chain variable region, the heavy chain variable region comprises amino acid sequences: (I) HCDR1, HCDR2 and HCDR3 respectively shown as SEQ ID NO: 1, SEQ ID NO: 2 and SEQ ID NO: 3; or (II) HCDR1, HCDR2 and HCDR3 as shown in SEQ ID NO: 11, 12 and 13 respectively, or (II) HCDR1, HCDR2 and HCDR3 as shown in SEQ ID NO: 12 and 13 respectively; or (III) HCDR1, HCDR2 and HCDR3 of which the amino acid sequences are respectively shown as SEQ ID NO: 21, 22 and 23; the light chain variable region comprises amino acid sequences: (I) an LCDR1, an LCDR2 and an LCDR3 respectively shown as SEQ ID NO: 4, 5 and 6; or (II) an LCDR1, an LCDR2 and an LCDR3 as shown in SEQ ID NO: 14, 15 and 16 respectively, or (II) an LCDR1, an LCDR2 and an LCDR3 as shown in SEQ ID NO: 16 respectively; or (III) LCDR1, LCDR2 and LCDR3 as shown in SEQ ID NO: 24, 35 and 26, respectively, as shown in SEQ ID NO: 16, SEQ ID NO: 24, SEQ ID NO: 35 and SEQ ID NO: 26. The neutralizing antibody YB9-258, the neutralizing antibody YB13-292 and the neutralizing antibody YB13-208 disclosed by the invention can be used for effectively inhibiting SARS-CoV-2 pseudovirus infection, and also has relatively good neutralizing activity on an SARS-CoV-2 true virus.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL GUANGZHOU MEDICAL UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com