Patents
Literature
70results about How to "No immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical compound micropore polysaccharide and application thereof
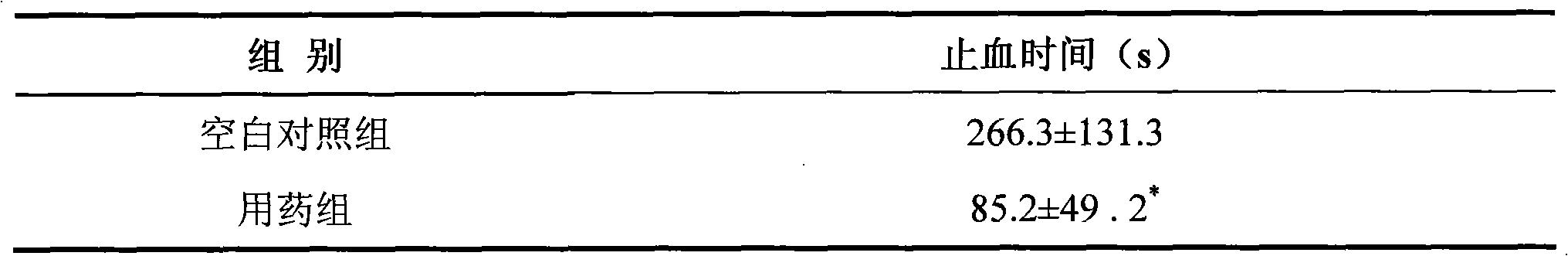
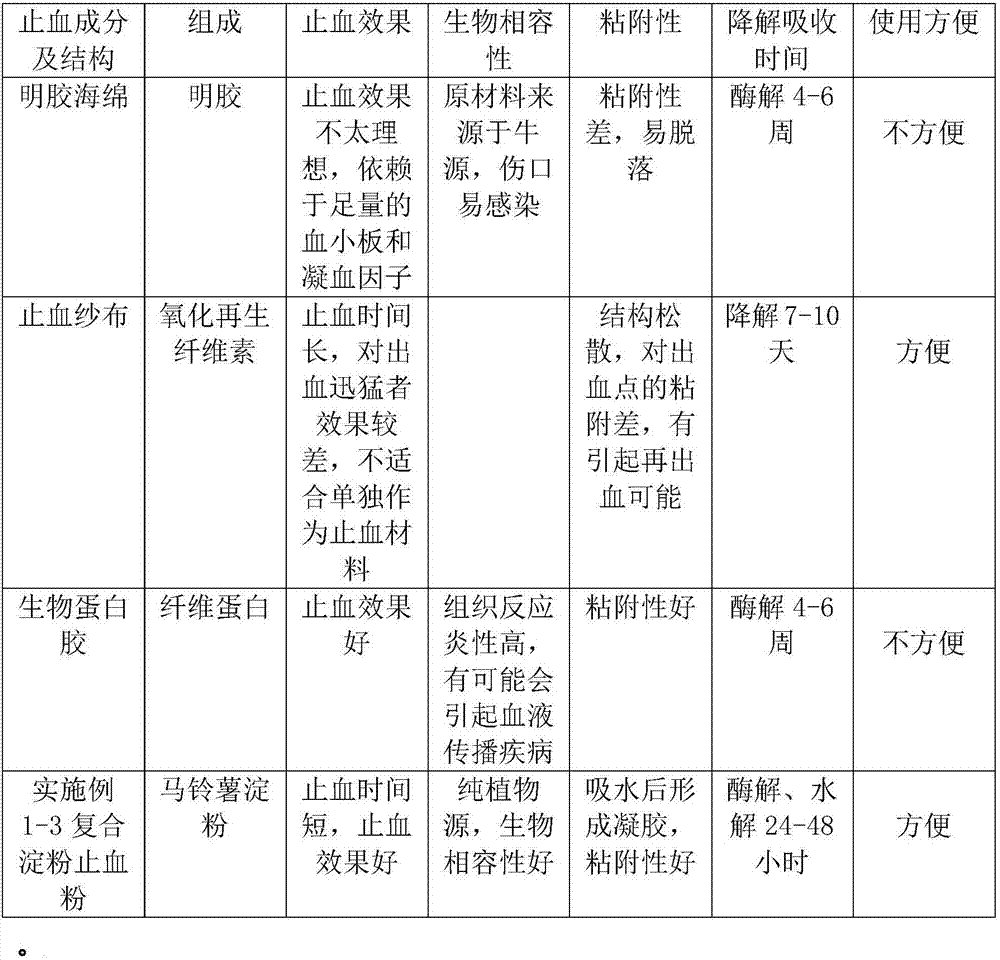
ActiveCN101584876AShort hemostatic timeAntigenicAbsorbent padsBandagesSurgical HemostasisWound surface
The invention relates to a medical compound micropore polysaccharide and application in hemostasis on wound surface bleeding areas of various wounds and operation tissues. The medical compound micropore polysaccharide is prepared by adopting a method with the following steps: (1) preparing starch fluid; (2) preparing carboxymethyl chitosan solution; (3) mixing the starch fluid and the carboxymethyl chitosan solution, and adding dispersant and emulsifier into the mixture; (4) emulsifying, crosslinking and co-polymerizing; and (5) refining, drying, packing and sterile treatment. The invention also discloses application of the medical compound micropore polysaccharide in preparing a hemostasis medicament used for the wound surface bleeding areas of wounds and operation tissues. The medical compound micropore polysaccharide of the invention has the following characteristics: 1, the hemostasis time is short, namely the hemostasis is generally finished in 3 to 5 minutes; 2, the medical compound micropore polysaccharide has no antigenicity, has polysaccharide components, and has no protein or immune reaction; 3, the medical compound micropore polysaccharide can be fully degraded and absorbed in vivo; 4, the medical compound micropore polysaccharide is a sterile product and is convenient to use by opening; and 5, the tissue reaction is light. Therefore, the medical compound micropore polysaccharide is a comparatively ideal surgical hemostasis product.
Owner:SAIKE SAISI BIOTECH CO LTD
Nanometer silver antibiotic powder fixed by silk fibroin and preparation method thereof
InactiveCN101044848AGood dispersionImprove stabilityBiocideAnimal repellantsReducing agentFood science
An antibacterial silk protein carried nano-Ag powder used for cosmetics, health-care food, enzyme immobilizing materials, biosensor, artificial skin or muscle, etc is prepared proportionally from silk protein and Ag nanoparticles. Its preparing process is also disclosed.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Engineered scaffold of amniotic membrane tissue and process for removing cells from aniotic membrane
InactiveCN1369555ASimple methodEasy for mass productionArtificial cell constructsVertebrate cellsChemistryMembrane configuration
An engineered scaffold of tissue is prepared from the amniotic membrane whose epicytes and fibrous matericytes has been removed. A process for removing said cells from amniotic membrane includes immersing the amniotic membrane in 0.2-5% trypsase solution at 37 deg.C for 20-25 min and stirring while flushing. Its advantages are no immunoreaction and simple process.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Monoclonal antibody capable of binding to specific discontinuous epitope occurring in ad1 region of human cytomegalovirus gb glycoprotein, and antigen-binding fragment thereof
The present invention provides a pharmaceutical composition for human cytomegalovirus HCMV causative of various disease states, the composition comprising a monoclonal antibody and an antigen-binding fragment thereof that specifically binds to the AD1 region of glycoprotein gB and that has excellent neutralizing capacity. The present invention provides: a monoclonal antibody and an antigen-binding fragment thereof having an excellent neutralizing capacity and cell-to-cell infection blocking capacity and specifically binding to a discontinuous sequence occurring in the HCMV AD1 region; a pharmaceutical composition comprising the antibody or the fragment thereof; and the like.
Owner:EVEC
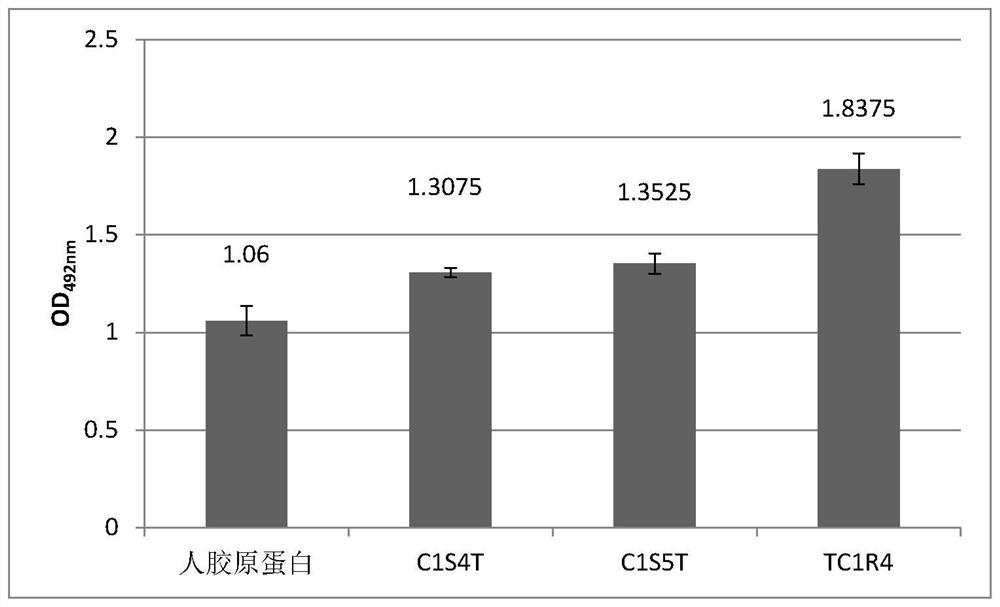
Recombinant I-type humanized collagen polypeptide as well as preparation method and application thereof
ActiveCN113621052AIncrease productionPromote cell adhesionCosmetic preparationsBacteriaCell adhesionProteinogenic amino acid
The invention discloses a recombinant I-type humanized collagen polypeptide as well as a preparation method and application thereof. The recombinant I-type humanized collagen polypeptide provided by the invention comprises n repeats of a sequence shown in SEQ ID No. 1, wherein n is an integer greater than or equal to 1, and when n is an integer greater than or equal to 2, the repeat sequences are directly connected; and optionally, the N tail end of the recombinant I-type humanized collagen polypeptide comprises an amino acid sequence which can be excised by TEV protease. The recombinant I-type humanized collagen polypeptide provided by the invention has the activity of promoting cell adhesion, the amino acid sequence of the recombinant protein is selected from a natural collagen amino acid sequence, and the recombinant protein does not generate an immune response when being applied to a human body; and moreover, the preparation method is simple, and high-yield collagen can be obtained at low cost.
Owner:SHANXI JINBO BIO PHARMA CO LTD
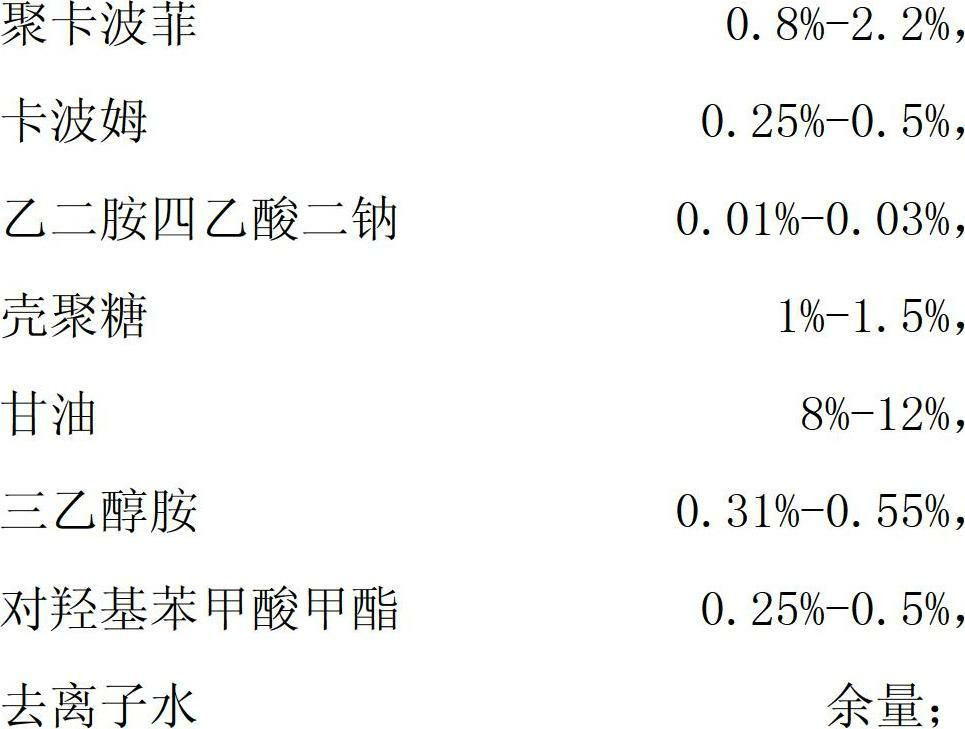
Vagina pH buffer antibacterial gel and preparation method thereof
InactiveCN102688182AImprove self-cleaning abilityReduce buildAntibacterial agentsAerosol deliveryAntigenDisease
The invention provides vagina pH buffer antibacterial gel, a preparation method thereof, and a using method thereof. The gel mainly comprises the components of polycarbophil, carbomer, ethylenediamine tetraacetic acid disodium, chitosan, glycerol, triethanolamine, methyl parahydroxybenzoats and deionized water. By using the weak acidity of polycarbophil and the antibacterial performance of chitosan, the gel can eliminate the source of recurrence of vagina inflammatory diseases and recovers the self-cleaning ability of the vagina. The gel is free from antigen performance, allergy and irritation, and convenient to use, and tissue reaction is avoided.
Owner:天津枫盛阳医疗器械技术股份有限公司
Non-virus nano nucleic acid transferring composite for curing gristle defection by injecting in joint cavity and preparing method thereof
InactiveCN101085357AGood biocompatibilityLow viscosityGenetic material ingredientsSkeletal disorderSolubilityNucleic acid transport
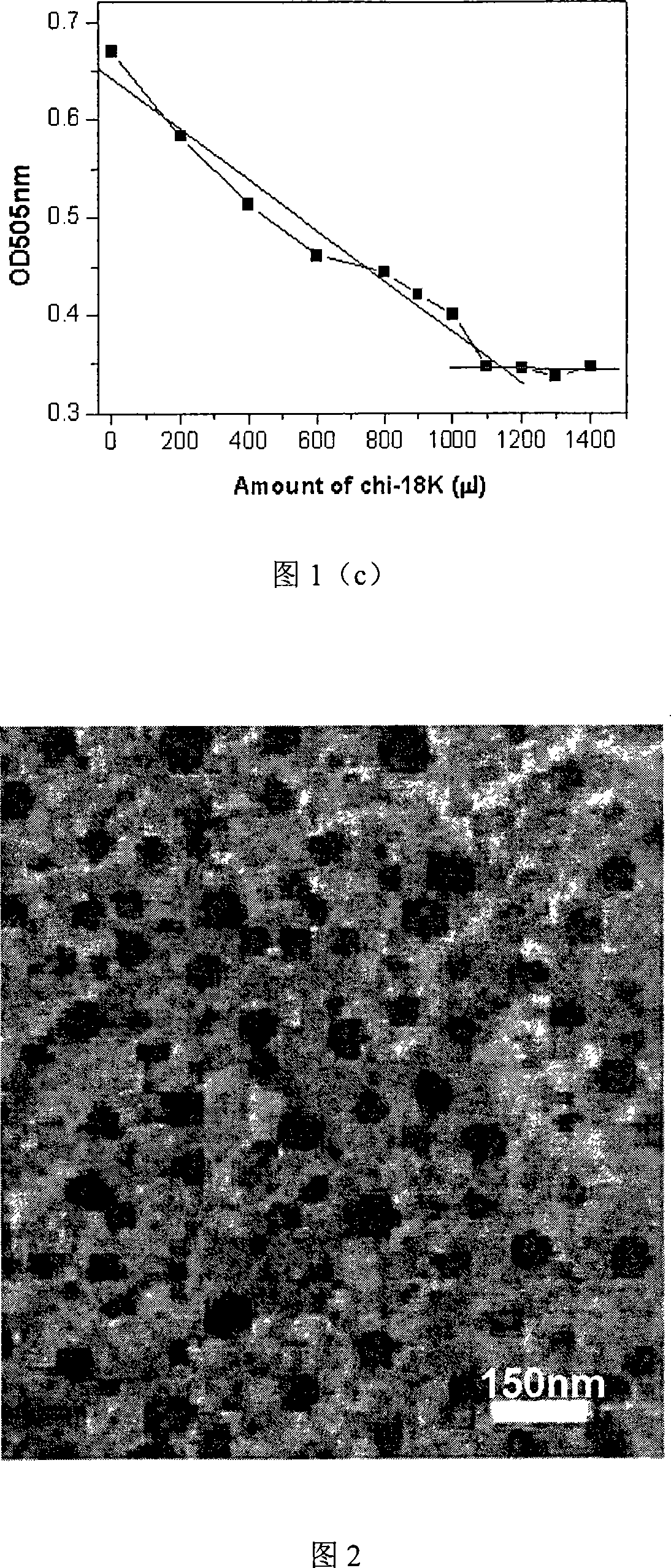
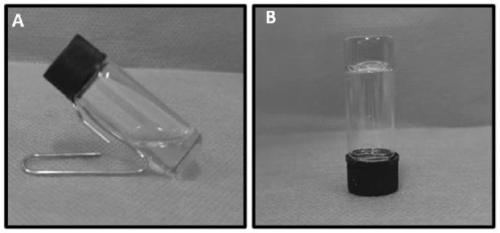
The invention discloses a non-viral nanometer nucleic acid transportation compound used for treating cartilage defect through intra-articular injection which comprises low-molecular chitosan with molecular weight of 5KD-100KDand percentage composition of amino group of 30-70%, and nucleic acid, wherein N / P of the compound is 0.2:1-10:1.The invention degrades high molecular chitosan to low molecular chitosan, increases water solubility of material, reduces viscosity of solution, reduces influence of sour environment of solvent to cell, improves compound size with nanometer, is in favor of performing genetic transmission to cartilago articularis cell, and protects target nucleic acid from neutralizing with synovial fluid and being degrade by nuclease in lysosome. The invention has simple preparation and abroad application scope; can solve engineering roadblock of cartilage repair with no requirement of operation, complex device and bracket material.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
Thiol/boronic group modified polymer, glucose-sensitive hydrogel composition, glucose-sensitive drug-loaded hydrogel, and preparation method thereof
ActiveCN110256669AStable mechanical strengthAdjust the release speedMetabolism disorderAerosol deliveryGlucose sensitivityConcentrations glucose
The invention relates to the field of macromolecules, in particular to a thiol / boronic group modified polymer, a glucose-sensitive hydrogel composition, glucose-sensitive drug-loaded hydrogel and a preparation method thereof. The thiol / phenylboronic group modified polymer is dispersed in a polysaccharide aqueous solution with an o-diol structure, and is stirred to swell under a near-neutral condition, so that the glucose sensitive hydrogel is obtained by utilizing spontaneous oxidation of the thiol group and condensation crosslinking of the boronic acid groups and the o-diol in the molecules. The preparation method of the glucose-sensitive hydrogel is simple, a condensing agent or a free radical initiator does not need to be added, and the glucose-sensitive hydrogel has good injectability, biocompatibility and glucose sensitivity. When the hydrogel is used as a drug carrier, only the drug needs to be added into the glucose sensitive hydrogel to be dissolved or uniformly dispersed. According to the prepared glucose-sensitive drug-loaded hydrogel, the release speed and release behavior of the drug can be adjusted by responding to the change of the glucose concentration, so that the drug can be quickly released when the glucose concentration is high.
Owner:WENZHOU MEDICAL UNIV
Anal fistula suppository and preparation method thereof
The invention provides an anal fistula biological suppository and a preparation method thereof. The anal fistula biological suppository uses small intestine submucosa tissues of inbred line animals without cell and DNA (deoxyribonucleic acid) components as the raw material, completely reserves the extracellular matrix component and structure, and has a micropore structure. The preparation method of the anal fistula biological suppository comprises the following operation steps: determination of animal source, pretreatment and rough cleaning of small intestine tissues, virus inactivation, cell removal, DNA removal treatment, formation, packaging and sterilization. The anal fistula biological suppository prepared by the method uses an inbred line animal as an animal source, and thus, the hereditary features are pure, stable and uniform, thereby radically ensuring the stability and uniformity of different batches of products; and the anal fistula biological suppository has fewer animal source DNA residues, completely reserves the three-dimensional structure of natural ECM, and has the advantages of low immune source property and high infection resistance.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Photosensitizer formula for photodynamically treating periodontitis, as well as preparation method and application thereof
InactiveCN106822894ANo side effectsNo immune responseAntibacterial agentsPhotodynamic therapyOral diseaseGel preparation
The invention discloses a photosensitizer formula for photodynamically treating periodontitis and an application thereof. A hydrogel preparation for treating periodontitis takes toluidine blue as a photoactive substance and carbopol as a gel. A formula for optimizing the gel preparation comprises the following components by mass concentration: 0.05%-3% of carbopol, 0.001%-1% of toluidine blue, 0.01%-0.4% of sodium hydroxide or 0.07%-2% of triethanolamine and the balance of water. A method for photodynamically treating periodontitis, provided by the invention, can be used for replacing an antibiotic therapy, so that the problem of antibiotics abuse can be prevented; when the preparation is used for treating the oral diseases caused by infections, such as, periodontitis, the antibacterial rate of once irradiation treatment can reach up to 99.99%; serious patients can be repeatedly treated at intervals; and the photosensitizer formula is free from toxic or side effect to normal organ tissues and has wide market prospects and high application values.
Owner:ZHEJIANG UNIV OF TECH
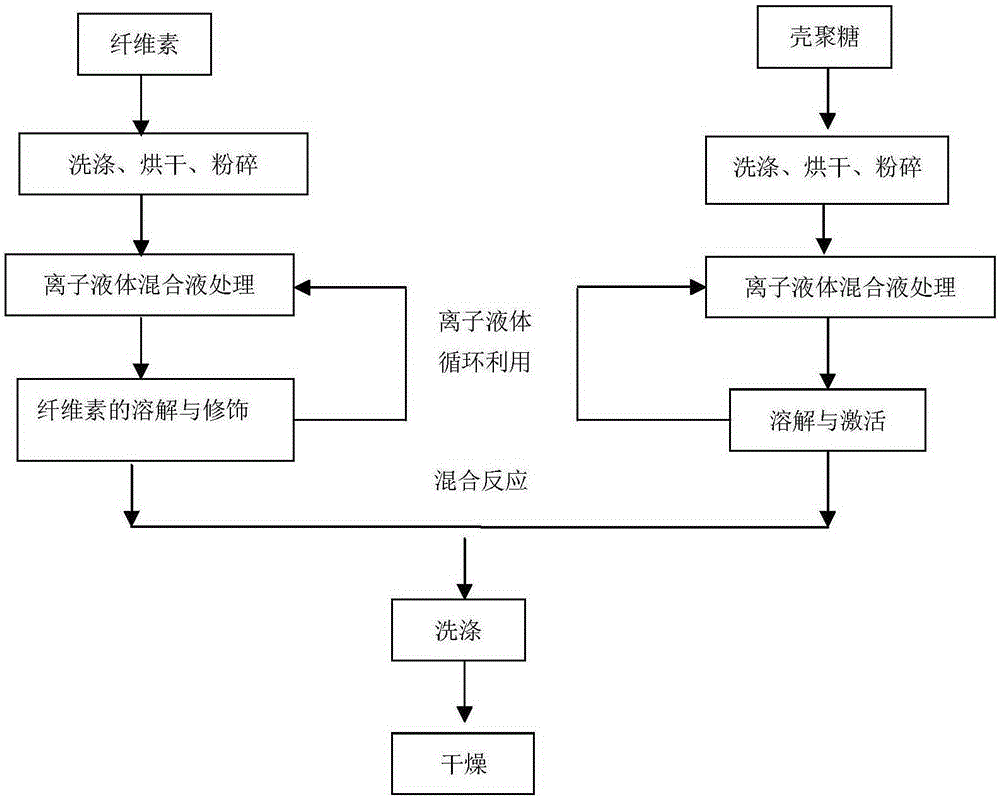
Preparation method of cellulose and chitosan hybrid fiber
ActiveCN105200557AImprove water retentionImprove thermal stabilityConjugated cellulose/protein artificial filamentsWater/sewage treatment by sorptionFiberIonic liquid
The invention discloses a preparation method of a cellulose and chitosan hybrid fiber. The preparation method comprises the following steps of (1) taking and drying cellulose, crushing to obtain cellulose powder; taking the mixed liquor of the cellulose powder and acidic ionic liquid, performing heat preservation, adding a carboxylation agent, stirring, and performing heat treatment; (2) taking and uniformly mixing the mixed liquor of the cellulose powder and the acidic ionic liquid, and performing heat treatment; (3) mixing the products of the step (1) and the step (2), heating, stirring and uniformly reacting; (4) adding a regeneration agent into an obtained sample to wash out liquid reagents such as acidic ionic liquid mixed liquor, and a solid is dried to obtain the hybrid fiber. The hybrid fiber obtained by the preparation method has the characteristics of good water-retaining property, high heat stability, antibiosis, environmental protection, ventilation, moisture prevention, porous property and the like. A reactant is stable in a reaction process, and by-products cannot be produced. The fiber has wide prospect in the fields of drug carriers, enzyme carriers, adsorbed substances and the like.
Owner:JIANGSU UNIV OF SCI & TECH
Preparation method of porcine frozen semen diluent
The invention discloses a preparation method of a porcine frozen semen diluent. The diluents comprises the following components by weight, and the preparation steps of the diluent includes: S1: material taking, S2: pH value adjustment, S3: fresh egg yolk adding, S4: centrifugation, S5: standing, and S6: filtration. The inositol compound employed in the invention can effectively reduce the formation of ice crystals formed in a semen freezing process, reduce the mechanical injury of ice crystals on sperms, and finally improve the motility rate, vitality, acrosome integrity, plasmolemma integrityand other technical indexes of porcine frozen semen, wherein penicillin and streptomycin mainly play the role of sterilization and microbial growth; monosaccharide is used as the main energy substance; citric acid and trihytdroxymethyl aminomethane interact to mainly serve as a buffer solution for decreasing sperm motility, thereby prolonging sperm lifetime and maintaining the fertilizing capability.
Owner:JILIN ACAD OF AGRI SCI
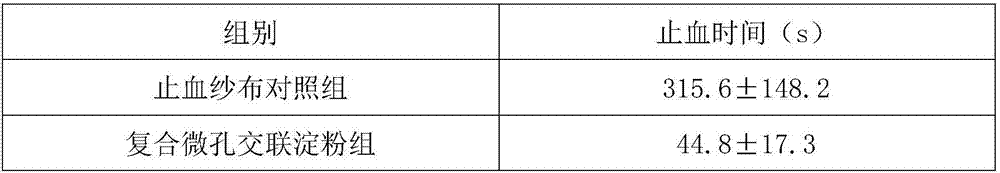
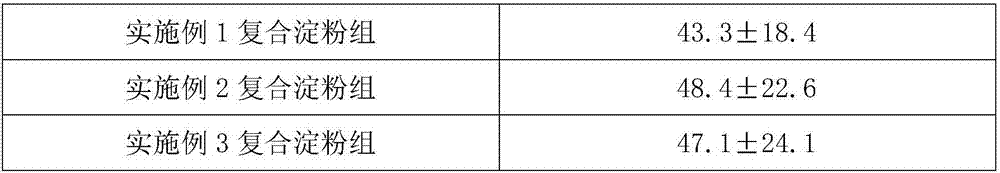
Composite starch styptic powder
InactiveCN106955370APromote activationNon-toxicSurgical adhesivesPharmaceutical delivery mechanismPotato starchParaffin oils
The invention provides composite starch styptic powder which is made of one of purified water, sweet potato starch, sodium alginate and sodium carboxymethylcellulose, an emulsifier and a dispersing agent as raw materials in an emulsification manner, wherein the emulsifier is selected from span and tween; and the dispersing agent is selected from paraffin or plant oil. The composite starch styptic powder provided by the invention has the technical effects that the powder is extracted from plant starch, when polysaccharide styptic granules are placed on bleeding wounds, moisture in blood can be absorbed by molecules of the granules, visible components of the blood are accumulated on the surfaces of the granules, then a gel mixture is formed, and the effect of stopping bleeding in time is achieved; meanwhile, activation of intrinsic coagulation factors can be accelerated, the intrinsic coagulation time can be shortened, partial sludged blood can be formed, and the stypticity action can be completed within tens of seconds.
Owner:JIANGSU HUANENG PHARMA
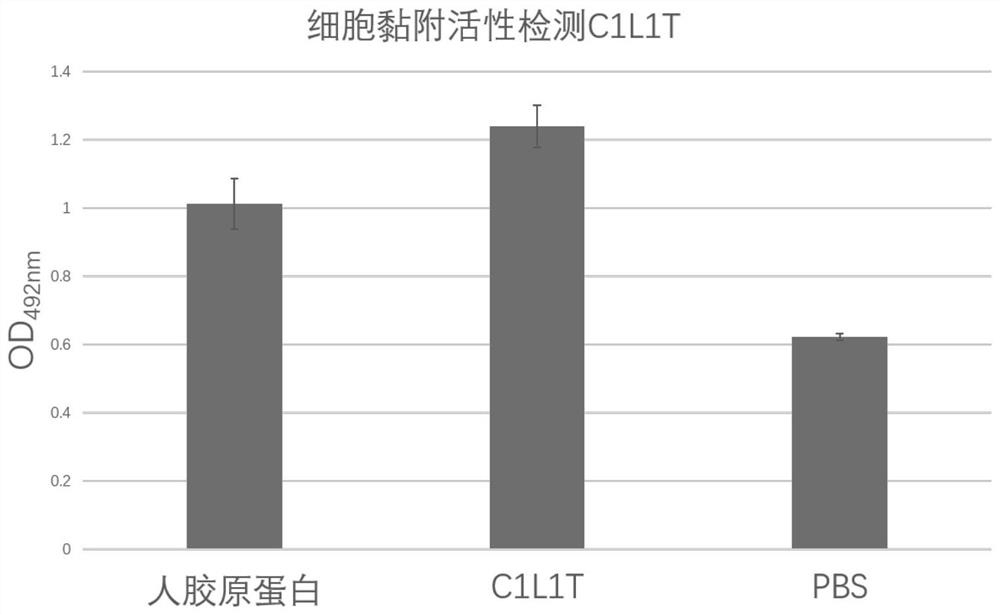
Recombinant I-type humanized collagen C1L1T and preparation method and application thereof
PendingCN113683680AIncrease productionPromote cell adhesionCosmetic preparationsBacteriaHuman bodyCell adhesion
The invention discloses recombinant I-type humanized collagen C1L1T and a preparation method and application thereof. The recombinant I-type humanized collagen C1L1T comprises a sequence shown as SEQ ID No. 3; optionally, the recombinant I-type humanized collagen C1L1T comprises a sequence shown as SEQ ID No. 2; and preferably, the sequence shown as SEQ ID No. 3 is directly connected with the sequence shown as SEQ ID No. 2. The recombinant I-type humanized collagen C1L1T has the advantages that the activity of promoting cell adhesion is realized, and the amino acid sequence of the recombinant I-type humanized collagen C1L1T is selected from the amino acid sequence of natural I-type human collagen; and when the recombinant I-type humanized collagen C1L1T is applied to a human body, the immune reaction is avoided; the preparation method is simple, and the collagen with higher yield can be obtained at low cost.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Preparation method for wharton jelly tissue engineering scaffold of human umbilical cord
InactiveCN109675114AImprove mechanical propertiesGood growthTissue regenerationProsthesisAdditive ingredientFreeze-drying
The invention discloses a preparation method for a wharton jelly tissue engineering scaffold of human umbilical cord. The preparation method specifically comprises the following steps: S1) separatingwharton jelly; removing blood vessels from fresh umbilical cord and then cutting into pieces; S2) mechanically smashing: adding wharton jelly cut in step S1 into normal saline, and then uniformly shaking into slurry. The invention relates to the technical field of biomedicine. The preparation method for the wharton jelly tissue engineering scaffold of human umbilical cord is capable of preparing the wharton jelly tissue engineering scaffold of human umbilical cord without cells by adopting cell-removing, freeze-drying and cross-linking processes; ingredients, such as glycosaminoglycan and II type collagen, in wharton jelly are remained in the prepared tissue engineering scaffold; cells inoculated onto the scaffold are adhered to scaffold duct walls and are under excellent growth state; substances are secreted; an in vivo test proves that no immunoreaction exists; the product has a bright application prospect, so that mechanical properties of scaffold are greatly promoted, antigenicityis effectively eliminated and a controllable degradation rate is achieved.
Owner:康泽生医学生物科技(武汉)有限公司
Hydrochloric acid ciprofloxacin lipidosome preparation and preparation method thereof
InactiveCN105232464AHigh encapsulation efficiencyLong retention timeAntibacterial agentsOrganic active ingredientsCholesterolPhosphate
The invention discloses a preparation method of a hydrochloric acid ciprofloxacin lipidosome preparation. The preparation method includes the steps that one part of hydrochloric acid ciprofloxacin is weighed, a phosphate buffer solution is added and stirred, and a hydrochloric acid ciprofloxacin solution is obtained; 2, blank liposome is prepared, wherein 10-50 parts of soya bean lecithin and 5-40 parts of cholesterol are weighed and mixed, chloroform is added and stirred for being dissolved, decompression is conducted for removing solvent, and a blank phospholipid membrane is prepared; 3, the membrane is dissolved through a small amount of chloroform, the hydrochloric acid ciprofloxacin solution obtained in step 1 and the phosphate buffer solution are taken for being added into an acetate solution, ultrasound treatment is conducted for 10-30 minutes through a bath type ultrasonic instrument at the temperature of 45-60 DEG C, a stable solution is formed, the obtained lipidosome solution is filtered twice through a microfiltration membrane, granulation is conducted, and the hydrochloric acid ciprofloxacin lipidosome preparation is obtained. The lipidosome produced through the ultrasonic instrument has the advantages that the particle size is small, particle size distribution is narrower, and the lipidosome has good preparation nature and target ability, can improve the curative effect, has a long-term effect, can reduce drug toxicity and can improve the drug stability.
Owner:ZHENGZHOU HOUYI PHARMA
Method for storing and refreshing FeiCheng Peaches using nitric oxide
InactiveCN101223909AExtended shelf lifeInhibition of diseaseFruit and vegetables preservationLoss rateCut flowers
The invention relates to a storage and preservation technology and method of Feicheng peaches taking a bio-active micro-molecular nitrogen monoxide as a material. The nitrogen monoxide material can be the mixed gas of nitrogen monoxide and inert gas or the water solution of nitrogen monoxide; the content of nitrogen monoxide in the mixed gas is 1 to 20 MulL<-1>, and the content of nitrogen monoxide in the water solution is 1 to 15 MumolL<-1>. The nitrogen monoxide is applied to fumigation of the Feicheng peaches while the water solution of nitrogen monoxide is applied to liquid immersion of fruits of the Feicheng peaches. The time of fumigation is 1-4h at the temperature of 15-27 DEG C, while the time used in liquid immersion of fruits is 1-20 min under the temperature of 15-27 DEG C. The storage time of fruits after the fumigation can be extended by 29 to 33 percent and the water loss rate is reduced by 21 to 24 percent, while the storage time of fruits after the liquid immersion can be extended by 60 to 80 percent and the water loss rate is reduced by 57 to 66 percent; meanwhile, the stored fruit causes no toxicity and harm to human bodies as well as no immune response. In addition, the method of the invention can be widely applied to the storage and preservation of fruits such as the Feicheng peaches, etc., and agricultural products of vegetables, fresh cut flowers, etc.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Preparation method of hemostatic material based on fibrin gel
InactiveCN107080857APromote formationAccelerates blood clotting processSurgical adhesivesPharmaceutical delivery mechanismFiberFibrin glue
The invention discloses a preparation method of a hemostatic material based on fibrin gel. The preparation method comprises the following steps: A. dissolution of bovine fibrinogen: at the temperature of 30-50 DEG C, bovine fibrinogen is dissolved by the use of a 0.9% NaCl solution so as to obtain a bovine fibrinogen solution with the concentration of 5-30 mg / Ml; B. dissolution of human thrombin: at the temperature of 30-50 DEG C, human thrombin is dissolved by the use of 330-660 mol / L of a CaCl2 solution so as to obtain a human thrombin solution with the concentration of 3-7 * 104 unit / L; and C. synthesis of gel: at the temperature of 30-50 DEG C, the fibrinogen solution obtained in the Step A and the thrombin solution obtained in the Step B are mixed according to the volume ratio of 1:1-5 so as to obtain mixed liquor; and the mixed liquor is dropped on gauze and then reacts on a shaker of 30-50 DEG C for 4-12 h. The hemostatic material prepared by the method has a good hemostatic effect, has good biocompatibility, can promote formation of granulation tissues and epithelial tissues and speed up healing and repairing of skin tissues, is beneficial to observation of wounds and healing situation, and is convenient for diagnosis and treatment of wounds.
Owner:SOUTHWEST JIAOTONG UNIV
Composite Moringa oleifera sugar polypeptide-amino acid buccal tablet and preparation method thereof
ActiveCN106858613AIncrease the rate of hydrolysisFacilitated releaseSugar food ingredientsNatural extract food ingredientsMoringa pterygospermaSugar
The invention discloses a composite Moringa oleifera sugar polypeptide-amino acid buccal tablet and a preparation method thereof. The buccal tablet comprises the following components in percentage by mass: 80-90% of Moringa oleifera protein peptide powder, 9-19% of sweetening agent and 0.5-1.5% of magnesium stearate, totaling 100%. The Moringa oleifera protein sugar polypeptide-amino acid product prepared by physical and enzymological techniques and a staged process. The molecular weight of the sugar polypeptides is 1-10KD, and the content is 17.5-21.7%; the amino acids have abundant varieties, and the content is enhanced to 6.33%; and the composite Moringa oleifera sugar polypeptide-amino acid buccal tablet has the effects of resisting oxidation, promoting mineral absorption and regulating the metabolic balance of the human body.
Owner:YUNNAN AGRICULTURAL UNIVERSITY
Neural stem cell microvesicles and application thereof
PendingCN110257333AUniform structureInhibit apoptosisPeptide/protein ingredientsNervous system cellsDiseaseMyocardial Reperfusion Injury
The invention relates to neural stem cell microvesicles and an application thereof, which belongs to the technical fields of biological materials and application; The microvesicles provided by the present invention are derived from neural stem cells, and the microvesicles specifically express and can deliver the protein HSP-70; through experiments, the application of the neural stem cell microvesicles in preparation of a drug for treating cardiomyocyte apoptosis-induced diseases such as myocardial reperfusion injury is proved, the above application is to increase the content of HSP-70 in cardiomyocytes by delivering HSP-70 through neural stem cell-derived microvesicles, so that cardiomyocyte apoptosis can be effectively inhibited; the use of the microvesicles of the present invention for the treatment of myocardial reperfusion injury not only has the same effect as stem cell therapy, but also avoids problems such as tumor formation, immunosuppression, and low survival rate during stem cell therapy, and growth factors, non-coding RNA and other cytoprotective factors are still available to help repair and regenerate damaged cells or tissues.
Owner:JIANGSU UNIV
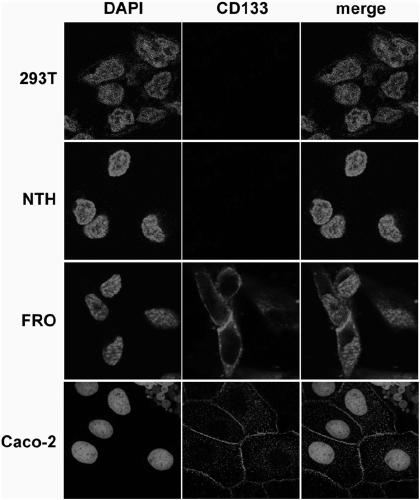
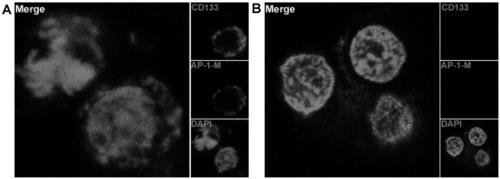
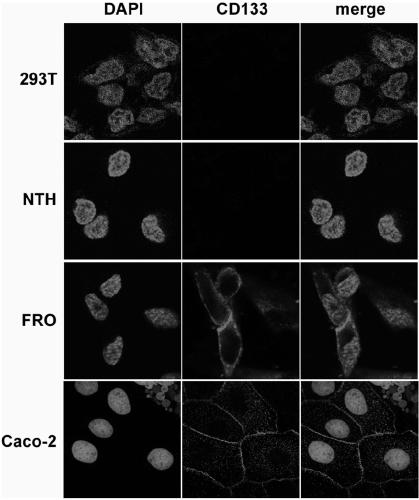
Drug conjugate of nucleic acid aptamer in targeted combination with CD133 protein and application of drug conjugate
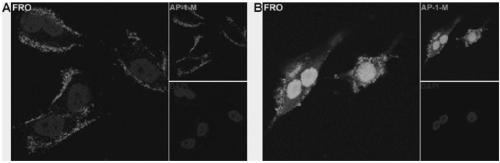
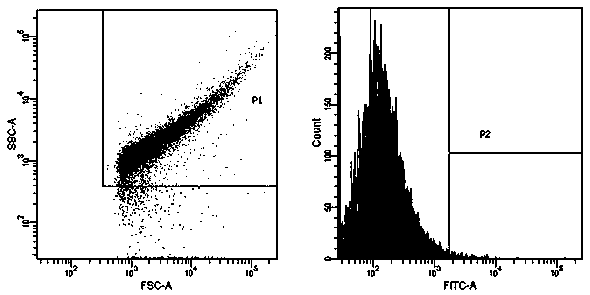
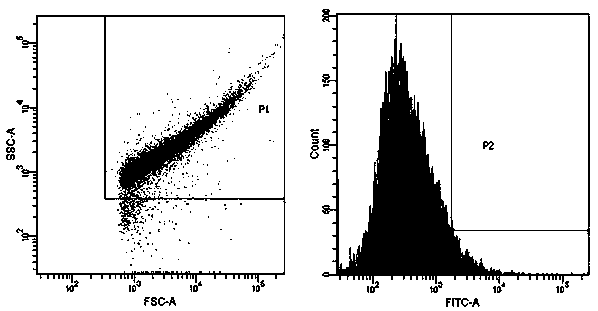
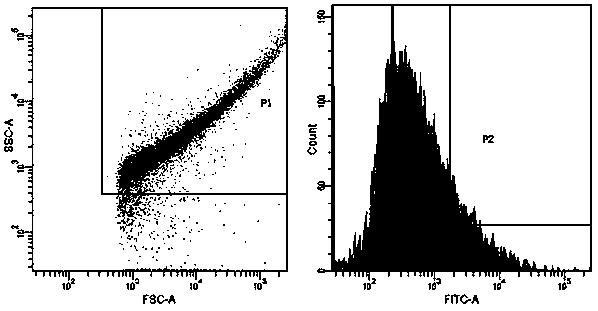
ActiveCN109439665AFast artificial synthesisLarge molecular weightOrganic active ingredientsPharmaceutical non-active ingredientsAptamerCytotoxicity
The invention provides a drug conjugate of a nucleic acid aptamer in targeted combination with a CD133 protein and an application of the drug conjugate. The sequence of the nucleic acid aptamer is shown in SEQ ID NO.1, the aptamer in targeted combination with the CD133 protein is screened from an ssDNA library through a cell screening method, and the adapter AP-1-M is chosen. In one scheme of thedrug conjugate, the classic chemotherapy drug adriamycin (DOX) and the AP-1-M are connected finally through a physical embedding mode, a nucleic acid aptamer-cellular poison conjugate (AP-1-M-DOC) intargeted combination with ATC is obtained, the cytotoxic effect of the nucleic acid aptamer-cellular poison conjugate (AP-1-M-DOC) in targeted combination with ATC is explored, a better target is provided for clinically screening and studying a novel targeted drug, and a novel method and idea is provided for clinical treatment of ATC.
Owner:ZHEJIANG CANCER HOSPITAL
A kind of preparation method of recombinant porcine epidemic diarrhea virus antibody
InactiveCN106146658BNo immune responseStrong targetingImmunoglobulins against virusesFermentationEscherichia coliAntigen
The present invention relates to a preparation method of a recombinant porcine epidemic diarrhea virus antibody. The preparation method of the recombinant porcine epidemic diarrhea virus antibody: select the mode of co-expression of antigen and antibody, and combine PEDV antigen gene S1 and anti-PEDV ScFv gene Co-expressed in the periplasmic cavity of Escherichia coli, three rounds of panning were performed by flow cytometry; the PEDV antibody was purified after expressing the ScFv gene in Escherichia coli, and the specificity of the PEDV ScFv antibody was detected by ELISA method, which was named PEDV‑ScFv; The construction of the anti-PEDV ScFv library and the preparation of the anti-PEDV antibody were carried out in two steps. The pig-derived PEDV antibody provided by the invention does not generate an immune response, is highly targeted, is easier to screen for antibodies with high neutralizing activity, and has a short preparation cycle and high speed.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Serum albumin nanoparticles combined with fluorescent dyes and coomassie brilliant blue as well as preparation method and application thereof in tumor diagnosis and treatment
ActiveCN108210923ASimple processStrong maneuverabilityPowder deliveryEnergy modified materialsChemistrySide effect
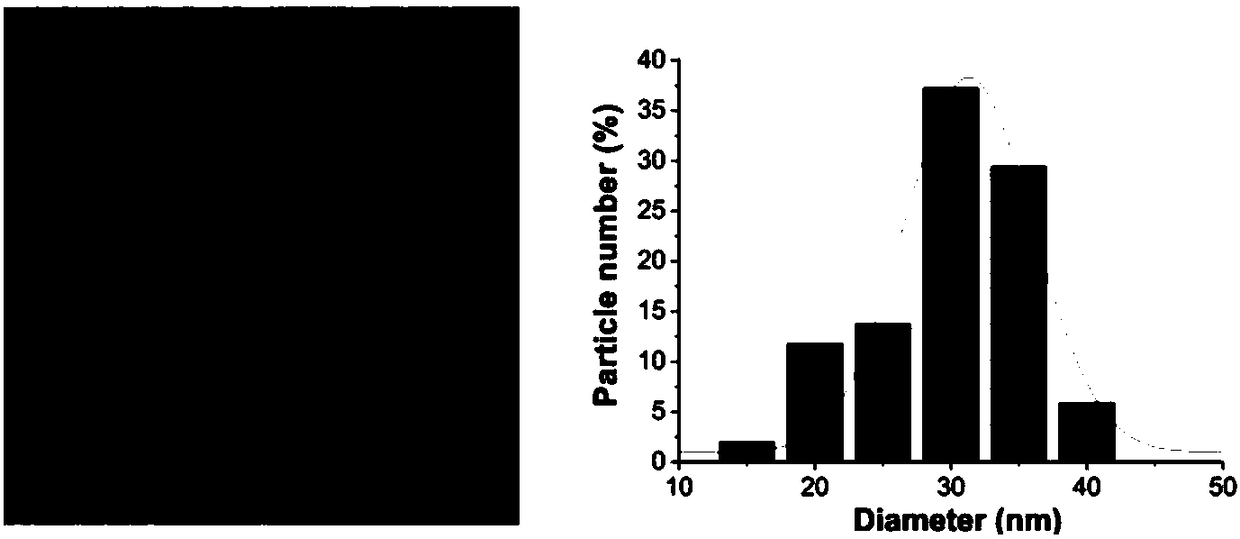
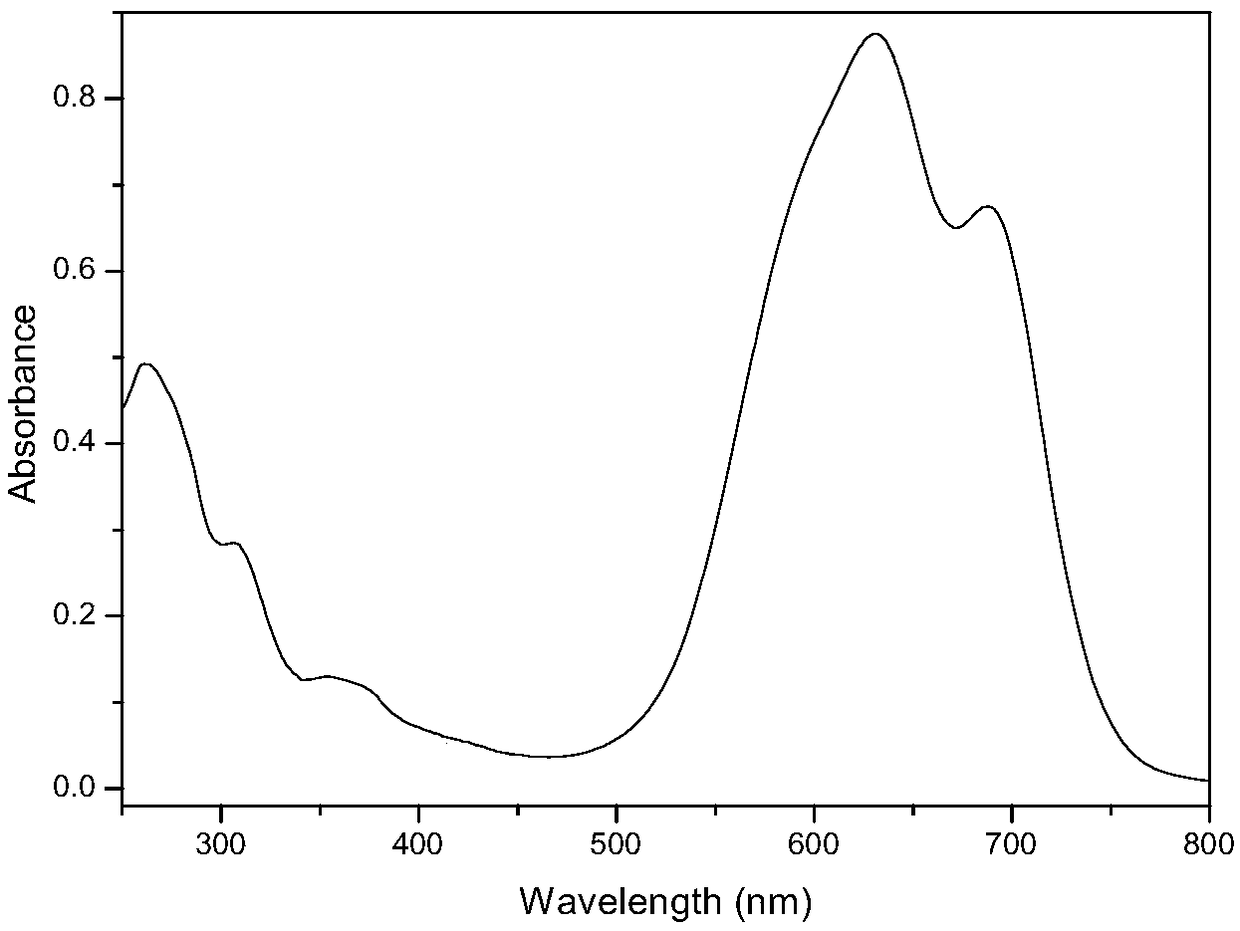
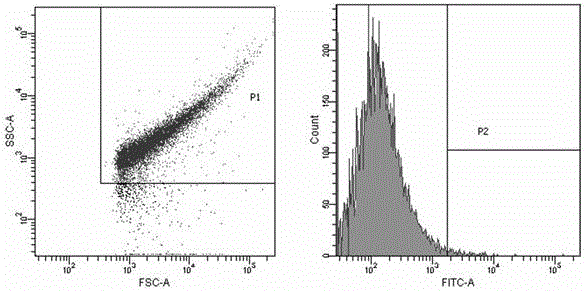
The invention discloses a preparation method of serum albumin (SA) nanoparticles based on fluorescent dyes (FD) and coomassie brilliant blue (CBB). The method comprises the following steps: S101: mixing a coomassie brilliant blue solution and a serum albumin solution at a mass ratio of (1 to 5) to (1 to 40), stirring and reacting for 0.5 to 15 hours; S102: post-filtering, washing, and dispersing to obtain an intermediate product; S103: adding the fluorescent dye, which accounts for 1 to 10% of the weight percentage of the intermediate product, to the intermediate product, stirring and reactingfor 0.5 to 15h; S104: filtering to obtain the serum albumin nanoparticles. The preparation method of the serum albumin nanoparticle of the invention has the advantages of simple process, strong operability and good process repeatability, and the prepared serum albumin nanoparticles combined with the fluorescent dyes and the coomassie brilliant blue have an excellent anti-tumor effect, high selectivity and small toxic and side effects.
Owner:HAINAN MEDICAL COLLEGE
Preparation method of recombinant porcine epidemic diarrhea virus antibody
InactiveCN106146658ANo immune responseStrong targetingImmunoglobulins against virusesFermentationEscherichia coliAntigen
The invention relates to a preparation method of a recombinant porcine epidemic diarrhea virus antibody, comprising: selecting an antigen antibody co-expression mode, co-expressing PEDV (porcine epidemic diarrhea virus) antigen gene and anti-PEDV ScFv gene in Escherichia coli periplasmic cavity, and performing triple sorting by using flow cytometry; using Escherichia coli to express the ScFv gene, then purifying the PEDV antibody, detecting specificity of the PEDV ScFv antibody by using ELISA (enzyme-linked immuno sorbent assay), and naming the antibody as PEDV-ScFv; wherein two specific steps are performed: constructing an anti-PEDV ScFv library and preparing the anti-PEDV antibody. The porcine-derived PEDV antibody provided herein never causes immune response, has high specificity, enables easier screening of an antibody with high neutralizing activity, and has short preparation cycle and high preparation speed.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
A nanoparticle preparation for treating HPV infection and preparation method thereof
ActiveCN105535994BHPV targeting is goodPromote absorptionOrganic active ingredientsPowder deliveryHuman papilloma virusSide effect
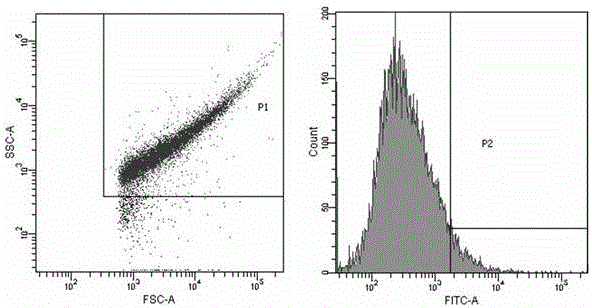
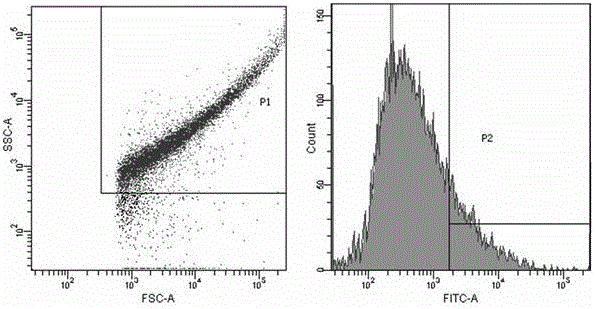
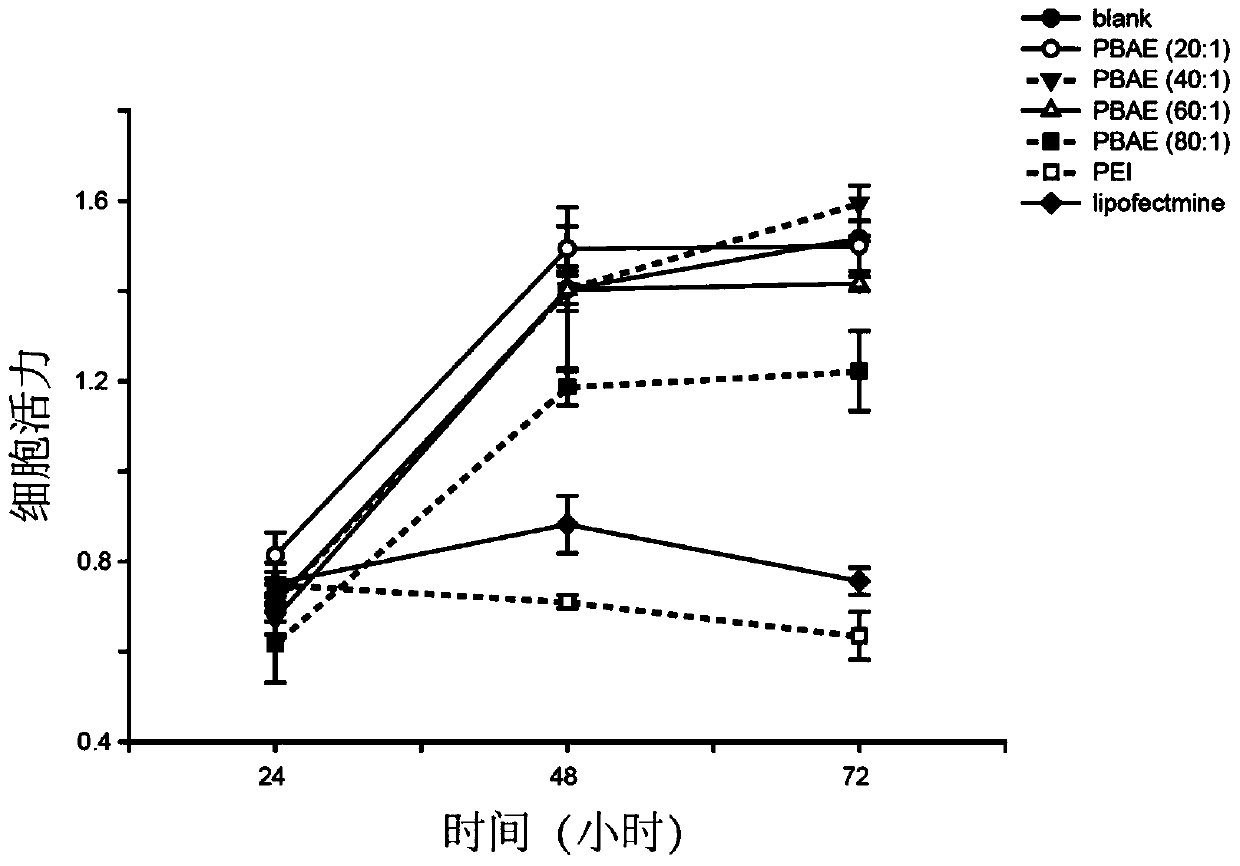
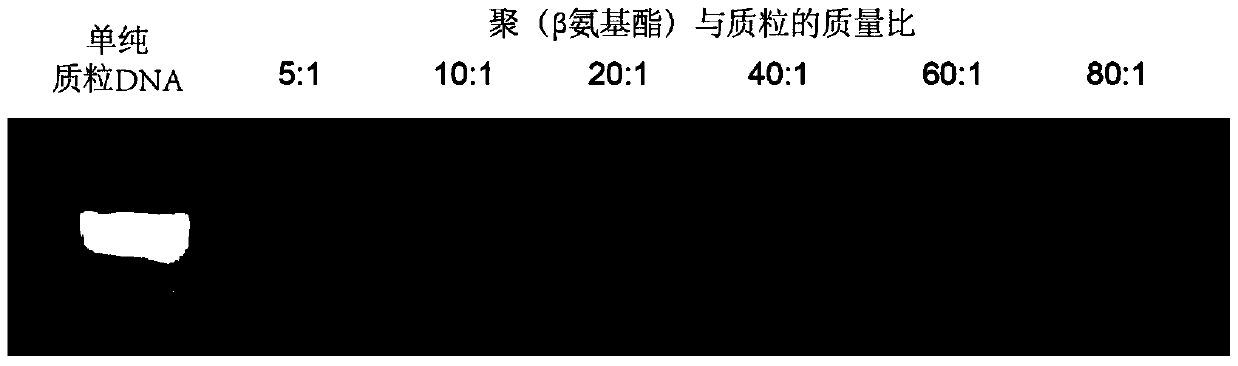
The invention relates to a nanoparticle preparation for treating HPV (Human Papilloma Virus) infection and a preparation method of the nanoparticle preparation. The nanoparticle preparation for treating the HPV infection is prepared from a poly(beta amino ester) polymer and a recombinant plasmid DNA (Deoxyribonucleic Acid) for knocking out or knocking down the HPV in a targeting manner. The nanoparticle preparation disclosed by the invention takes the poly (beta amino ester) polymer and the recombinant plasmid DNA for knocking out or knocking down the HPV in a targeting manner as main raw materials and has the advantages of good specificity, high transfection efficiency, low toxic or side effects and the like.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Nanometer silver antibiotic powder fixed by silk fibroin and preparation method thereof
An antibacterial silk protein carried nano-Ag powder used for cosmetics, health-care food, enzyme immobilizing materials, biosensor, artificial skin or muscle, etc is prepared proportionally from silk protein and Ag nanoparticles. Its preparing process is also disclosed.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Hybridoma cells capable of secreting substances resisting PEDV (porcine epidemic diarrhea virus) monoclonal antibodies, monoclonal antibody and application
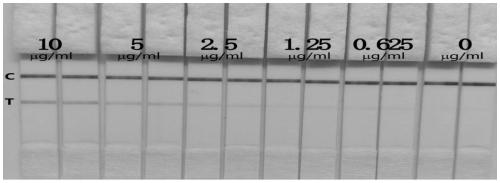
ActiveCN110144330AAccurate detectionSensitive detectionBiological material analysisImmunoglobulins against virusesEpidemic diarrheaTiter
The invention provides hybridoma cells capable of secreting substances resisting PEDV (porcine epidemic diarrhea virus) monoclonal antibodies, a monoclonal antibody and an application. The hybridoma cells are respectively named as 4A11 and 3H7, are preserved in the China Center for Type Culture Collection, in Wuhan University, Wuhan, China on 13 June 2018, with the preservation numbers being CCTCCNO:C2018123 and CCTCC NO:C2018122. The hybridoma cell strains 4A11 and 3H7 can secrete monoclonal antibodies resisting porcine epidemic diarrhea viruses at a high yield, and ascites indirect-ELISA titer can reach 10<-6>. The porcine epidemic diarrhea virus detection test paper is also established by using the monoclonal antibody as a core, so that the porcine epidemic diarrhea viruses can be highly-specifically, accurately and sensitively detected, the operation is simple and convenient, effective and rapid site fast detection can be realized, and material support and technical support are provided for detection, diagnosis and scientific guidance on prevention and control of the porcine epidemic diarrhea viruses in China.
Owner:JINAN UNIVERSITY
Monocyte source exosome preparation applied to osteogenic differentiation of mesenchymal stem cells
PendingCN110951685AGood biocompatibilityNon-toxicCell dissociation methodsSkeletal/connective tissue cellsOsseous DifferentiationDisease
The invention relates to a monocyte source exosome preparation applied to osteogenic differentiation of mesenchymal stem cells. The monocyte source exosome preparation comprises exosomes secreted by monocytes in human peripheral blood and PBS (Phosphate Buffer Solution); a preparation method comprises the following steps: culturing monocytes sorted in the human peripheral blood at 37 DEG C until the monocytes grow to about 80%; the replacing with a complete medium without exosomes fetal bovine serum, and collecting the culture medium after culturing for 48 h; carrying out centrifugal treatmenton the culture medium; and storing 1xPBS resuspended sediment at -80 DEG C, so as to obtain the monocyte source exosome preparation. In view of a pathophysiological progress involving orthopedic diseases, the exosome preparation prepared from the exosomes secreted by the monocytes in the human peripheral blood has the properties of very good biocompatibility, no toxicity and no immune response, and can replace traditional osteogenic inducers, so that unknown risks caused by in-vivo injection are avoided and a novel treatment application is provided for bone defect, osteonecrosis, osteoporosis, bone metabolic diseases and the like on clinic.
Owner:TIANJIN KANGTING BIOLOGICAL ENG GRP CO LTD
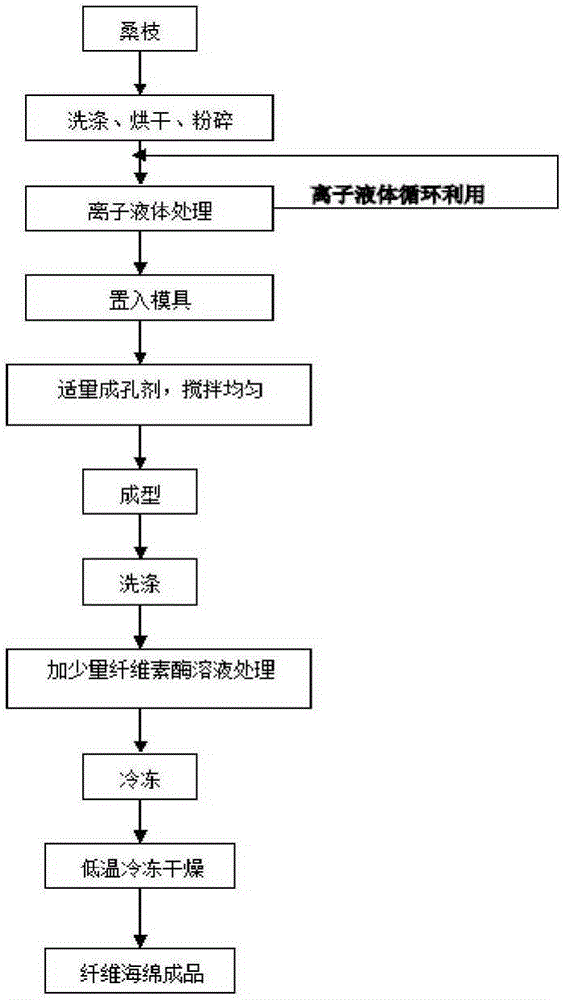
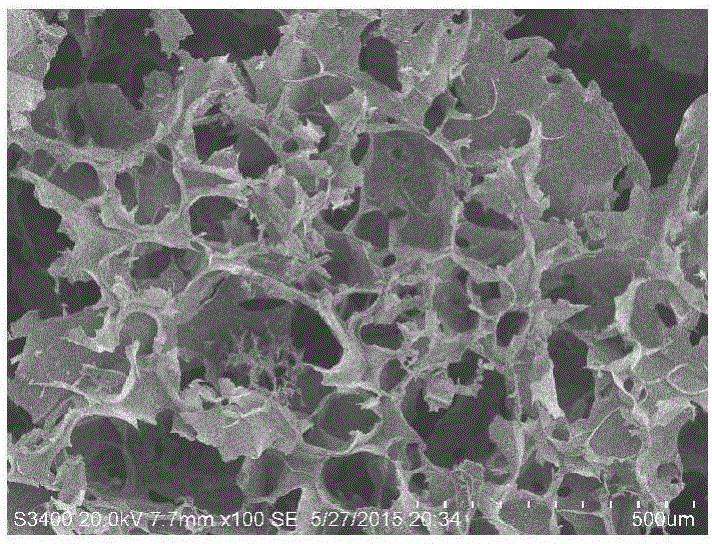
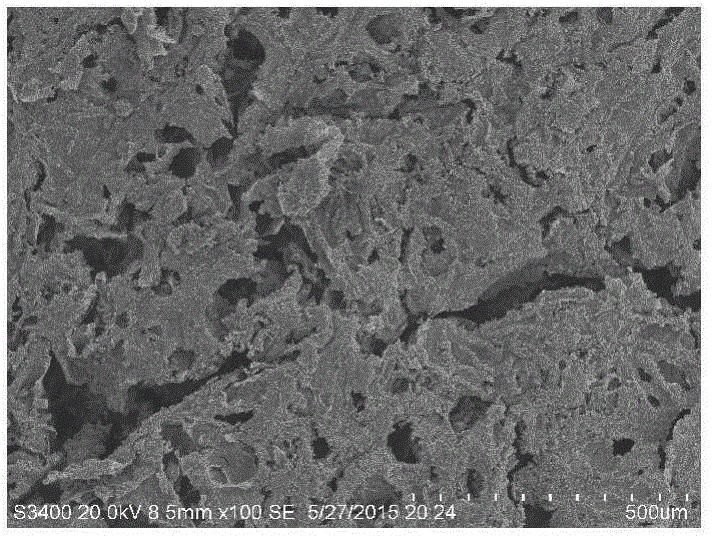
Preparation method for antibacterial cellulose sponge material
The invention discloses a preparation method for an antibacterial cellulose sponge material. The method comprises the following steps: (1) washing mulberry twigs, drying and grinding the mulberry twigs to certain size, thereby obtaining mulberry powder; (2) taking certain amount of mulberry powder to mix with certain amount of a prepared ionic liquid into a three-necked flask, and heating in a heat-collection type constant-temperature heating magnetic stirrer; (3) putting a sample obtained in step (2) into a mold, and adding an appropriate amount of a pore-forming agent while uniformly stirring and molding; (40 washing to remove the pore-forming agent and the remaining solvent, adding a prepared cellulose solution into the mold to react; and (5) freezing the molded sample, and carrying out low-temperature freeze-drying on the frozen molded sample, thereby obtaining a finished product of the cellulose sponge. According to the method disclosed by the invention, the renewable mulberry twig waste is taken as the raw material, and recycling is fast, thereby avoiding environmental pollution caused by burning the mulberry twigs, and realizing high value conversion of the mulberry twig waste; and the antibacterial cellulose sponge material has the characteristics such as gas permeability, water retention, moisture retention and the like, and has the advantages of being free of allergens and immune response, having natural antibacterial effect and the like.
Owner:浙江省仙居县南方海绵有限公司
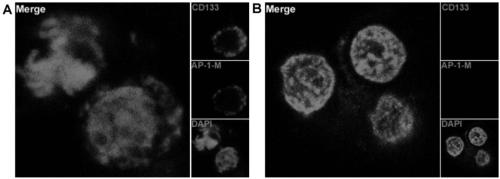
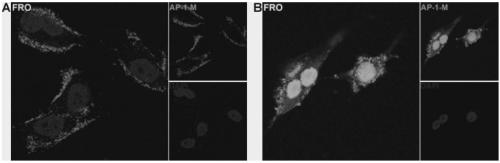
Aptamer for performing target combination on CD133 albumen as well as screening method and application thereof
ActiveCN109536503AFast artificial synthesisLarge molecular weightOrganic active ingredientsPharmaceutical non-active ingredientsAptamerImmunofluorescence
The invention provides an aptamer for performing target combination on CD133 albumen as well as a screening method and application thereof. A sequence of the aptamer is shown in SEQ ID NO.1. Accordingto the aptamer disclosed by the invention, by adopting a cell screening method, the aptamer for performing target combination on CD133 albumen is screened out from an ssDNA library, the screened ssDNA is detected through a high-throughput sequencing method, and finally, the stability and the compatibility of the obtained sequence can be analyzed through a method of flow cytometry, and then the aptamer AP-1-M is picked out. An immunofluorescence method proves that the aptamer AP-1-M can specially perform targeted combination on the CD133 albumen, and with the combination of the aptamer AP-1-Mand ATC cells for expressing the CD133 albumen, a targeted delivery effect can be achieved, and a new thinking and method can be provided for the targeted treatment on the ATC.
Owner:ZHEJIANG CANCER HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com