Recombinant I-type humanized collagen polypeptide as well as preparation method and application thereof
A collagen polypeptide and humanized technology, applied in the field of genetic engineering, can solve the problems of long cycle time, high production cost of human collagen, and inability to produce large-scale production, and achieve the effect of simple preparation method and good cell adhesion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of recombinant type I humanized collagen TC1R4
[0058] 1. Construction of TC1R4 gene expression vector
[0059] The full-length protein sequence of the recombinant type I humanized collagen TC1R4 used in this example is the amino acid sequence shown in SEQ ID No.3, with a full length of 240 aa. In order to purify the TC1R4 protein after it is expressed, the N-terminal of the amino acid sequence shown in SEQ ID No.3 is connected with the amino acid sequence shown in SEQ ID No.4 to form the amino acid sequence shown in SEQ ID No.5 , its full length is 246aa. The coding sequence corresponding to the amino acid sequence was codon-optimized for the codons of Escherichia coli, and the corresponding gene has a full length of 738 bp (see the underlined sequence in SEQ ID No. 6 for details). In order to facilitate the construction of subsequent expression vectors, a restriction site sequence was added to the 5' end of the coding sequence, and a stop cod...
Embodiment 2
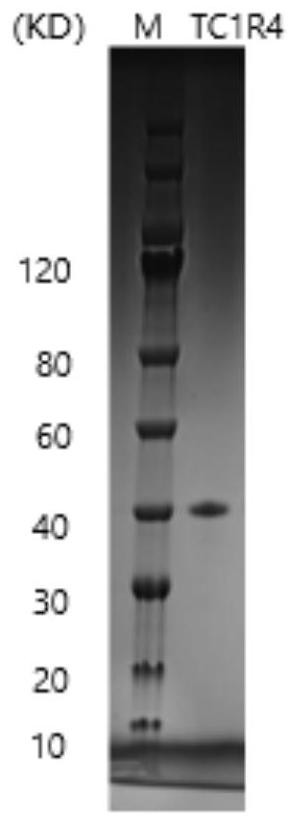
[0071] Example 2: Mass Spectrometric Detection of Recombinant Type I Humanized Collagen TC1R4
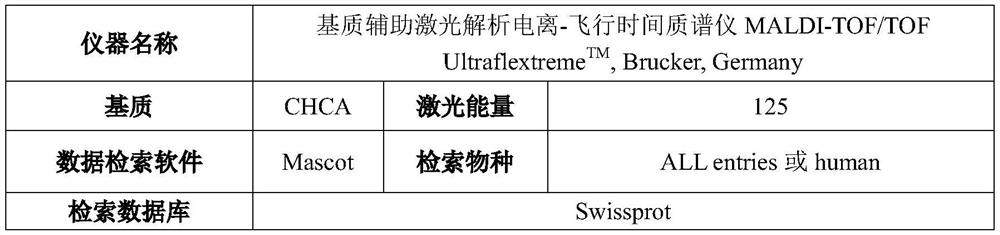
[0072] Mass spectrometry detection of TC1R4 protein was performed according to the conditions shown in Table 1.
[0073] Table 1 Conditions for mass spectrometry detection
[0074]
[0075]TC1R4 protein samples were treated with DTT reduction and iodoacetamide alkylation, and then digested with trypsin overnight. The peptides obtained after enzymatic hydrolysis were desalted by C18ZipTip, mixed with matrix α-cyano-4-hydroxycinnamic acid (CHCA) and plated. Finally, matrix-assisted laser desorption ionization-time-of-flight mass spectrometer MALDI-TOF / TOFUltraflextremeTM, Brucker, Germany was used for analysis (for the technique of peptide fingerprinting, please refer to: ProteinJ.201635:212-7).
[0076] Database retrieval was handled from the Peptide Mass Fingerprint page on the local mascot website. The protein identification results were obtained based on the primary mass spe...
Embodiment 3
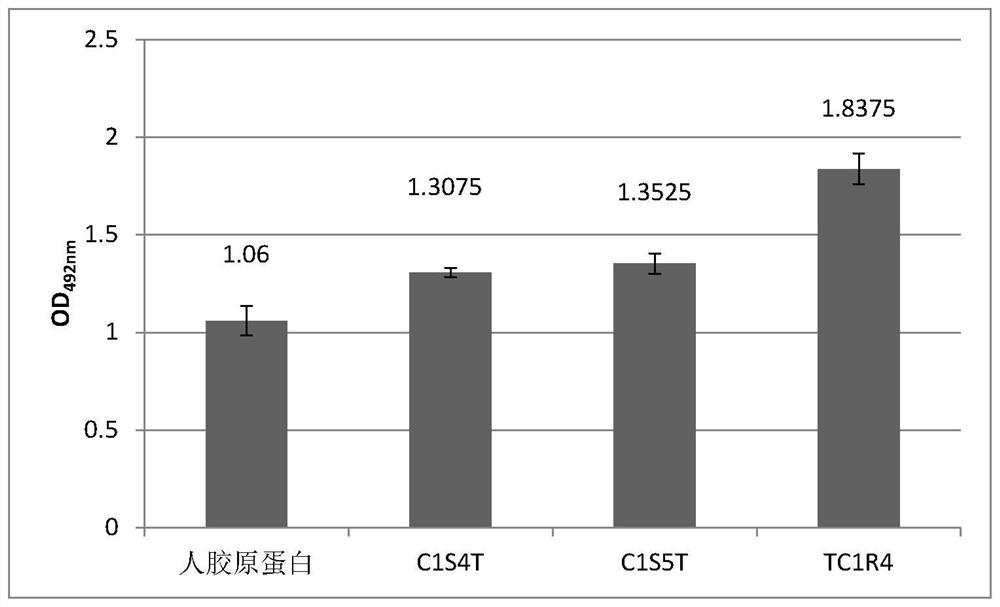
[0083] Example 3: Bioactivity Detection of Recombinant Type I Humanized Collagen TC1R4
[0084] Collagen activity detection method can refer to the literature Juming Yao, Satoshi Yanagisawa, Tetsuo Asakura, Design, Expression and Characterization of Collagen-Like Proteins Based on the Cell Adhesive and Crosslinking Sequences Derived from Native Collagens, J Biochem.136, 643-649 (2004). The specific implementation method is as follows:
[0085] (1) Use the ultraviolet absorption method to detect the concentration of the protein sample to be tested, including commercial human collagen (Sigma, C7774), recombinant type I humanized collagen TC1R4 provided by the present invention, recombinant type III collagen C1S4T, recombinant Type III collagen C1S5T (for the sequences of the proteins C1S4T and C1S5T, please refer to the patent application with application number 201811254050.7).
[0086] Specifically, the UV absorption of samples at 215nm and 225nm was measured respectively, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com