Medical compound micropore polysaccharide and application thereof
A polysaccharide and microporous technology, applied in medical science, bandages, absorbent pads, etc., can solve the problems of slow water absorption, difficult degradation, poor adhesion, etc., and achieve the effect of light tissue reaction and short hemostasis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
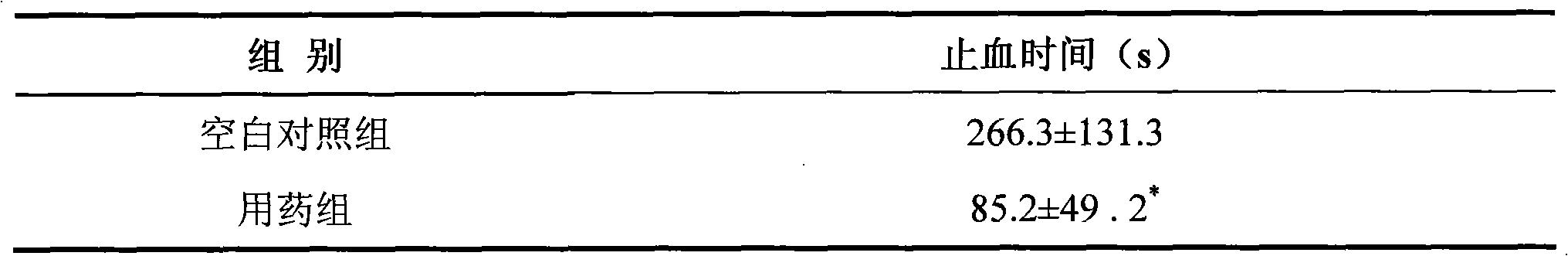
Image
Examples
Embodiment 1
[0025] Example 1: Preparation method of medical composite microporous polysaccharide
[0026] 1) Preparation of starch liquid
[0027] Accurately weigh 10.0 g of starch, measure 100 ml of distilled water with a measuring cylinder, add it to the starch, and heat it in a water bath until it becomes a paste.
[0028] 2) Dissolution of carboxymethyl chitosan
[0029] Weigh 1.0 g of carboxymethyl chitosan, add it to 500 ml of water with stirring, and dissolve it to clarify.
[0030] 3) Mix
[0031] ①Add the carboxymethyl chitosan solution to the starch solution and stir evenly. Adjust the pH to 9.0-10.0 with the prepared NaOH solution; ② Adjust the temperature of the water bath of the reactor to 60°C, add 500ml of liquid paraffin, stir, add 5.0g of Span 60 emulsifier, and after the solution reaches 60°C, add the above mixture, stirring vigorously.
[0032] 4) Emulsifying cross-linking copolymerization
[0033] Add 2ml of epichlorohydrin, react for 5h, stop stirring, and disch...
Embodiment 2
[0037] 1) Preparation of starch liquid
[0038] Accurately weigh 10.0 g of starch, measure 200 ml of distilled water with a measuring cylinder, add it to the starch, and heat it in a water bath until it becomes a paste.
[0039] 2) Dissolution of carboxymethyl chitosan
[0040] Weigh 0.1 g of carboxymethyl chitosan, add 10 ml of distilled water under stirring, dissolve and clarify.
[0041] 3) Mix
[0042] ①Add the carboxymethyl chitosan solution to the starch solution and stir evenly. Adjust its pH to 9.0 with the prepared NaOH solution and add 5ml of epichlorohydrin; ② adjust the temperature of the water bath of the reactor to 70°C, add 250ml of liquid paraffin, stir, add 10.0g of Span 80 emulsifier, and wait for the solution to reach 70 After ℃, the above mixture was added and stirred vigorously.
[0043] 4) Emulsifying cross-linking copolymerization
[0044] Add 15ml of formaldehyde, react for 10h, stop stirring, and discharge.
[0045] 5) Refined
[0046] After the...
Embodiment 3
[0047] Example 3: Preparation method of medical composite microporous polysaccharide
[0048] 1) Preparation of starch liquid
[0049] Accurately weigh 10.0 g of starch, measure 50 ml of distilled water with a measuring cylinder, add it to the starch, and heat it in a water bath until it becomes a paste.
[0050]2) Dissolution of carboxymethyl chitosan
[0051] Weigh 0.3 g of carboxymethyl chitosan, add 100 ml of distilled water under stirring, dissolve and clarify.
[0052] 3) Mix
[0053] ①Add the carboxymethyl chitosan solution to the starch solution and stir evenly. Adjust its pH to 7.0-8.0 with the prepared NaOH solution, and add 1.0 ml of formaldehyde; ② adjust the water bath temperature of the reactor to 65°C, add 1000ml of petroleum ether, stir, add 15g of Tween 60 emulsifier, and wait for the solution to reach 65 ℃, add the above mixture and stir vigorously.
[0054] 4) Emulsifying cross-linking copolymerization
[0055] The reaction was carried out for 3h, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com