Thiol/boronic group modified polymer, glucose-sensitive hydrogel composition, glucose-sensitive drug-loaded hydrogel, and preparation method thereof
A glucose-sensitive, thiol-modified technology, applied in the field of polymers, can solve the problems of inability to form a three-dimensional gel network, unfavorable biopharmaceutical loading, and limited drug release performance, and achieve good glucose sensitivity and good glucose-responsive drug release. , will not produce the effect of immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Polymer modified by mercapto / boronic acid groups
[0042] According to the feed listed in Table 1, 100 mg γ-polyglutamic acid (γ-PGA) was dissolved in MES (pH=6) solution, and its side chain carboxyl group was first activated by EDC / NHS in the dark for 1 hour, and then cysteine hydrochloride was added Acid (Cys) reacted at room temperature for 6h. Then the reaction solution was dialyzed in deionized water for 72 hours with a dialysis bag with a molecular weight of 7 kDa to remove the condensing agent and freeze-dried to prepare the thiol-modified polymer; weigh the thiol-modified polymer (PGA-Cys) and dissolve it in the MES solution, Its side chain carboxyl group was first activated with EDC / NHS in the dark for 0.5h, then added 3-aminophenylboronic acid dissolved in 1N dilute hydrochloric acid and reacted in the dark for 72h at room temperature, dialyzed with a dialysis bag with a molecular weight of 7kDa for 72h, removed the condensing agent, and freeze-dri...
Embodiment 2
[0048] The preparation of embodiment 2 glucose-sensitive hydrogels
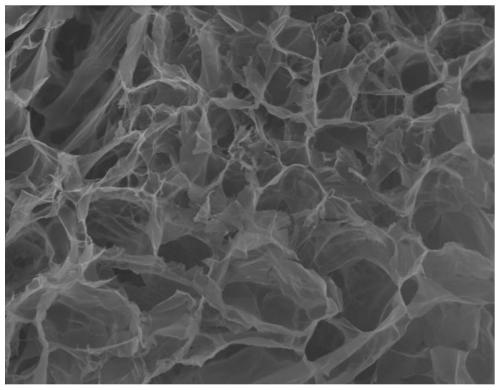
[0049] According to the formula listed in Table 2, weigh the o-diol polysaccharide and dissolve it completely in a certain volume of distilled water to form a clear solution, adjust its pH to 8.0 with 1N NaOH; add the polymer powder modified by thiol / boronic acid group directly Into the polysaccharide solution prepared above, stirring overnight to swell, its sulfhydryl group spontaneously oxidizes and crosslinks, and its boronic acid group condenses with adjacent diols to form a glucose-sensitive hydrogel.
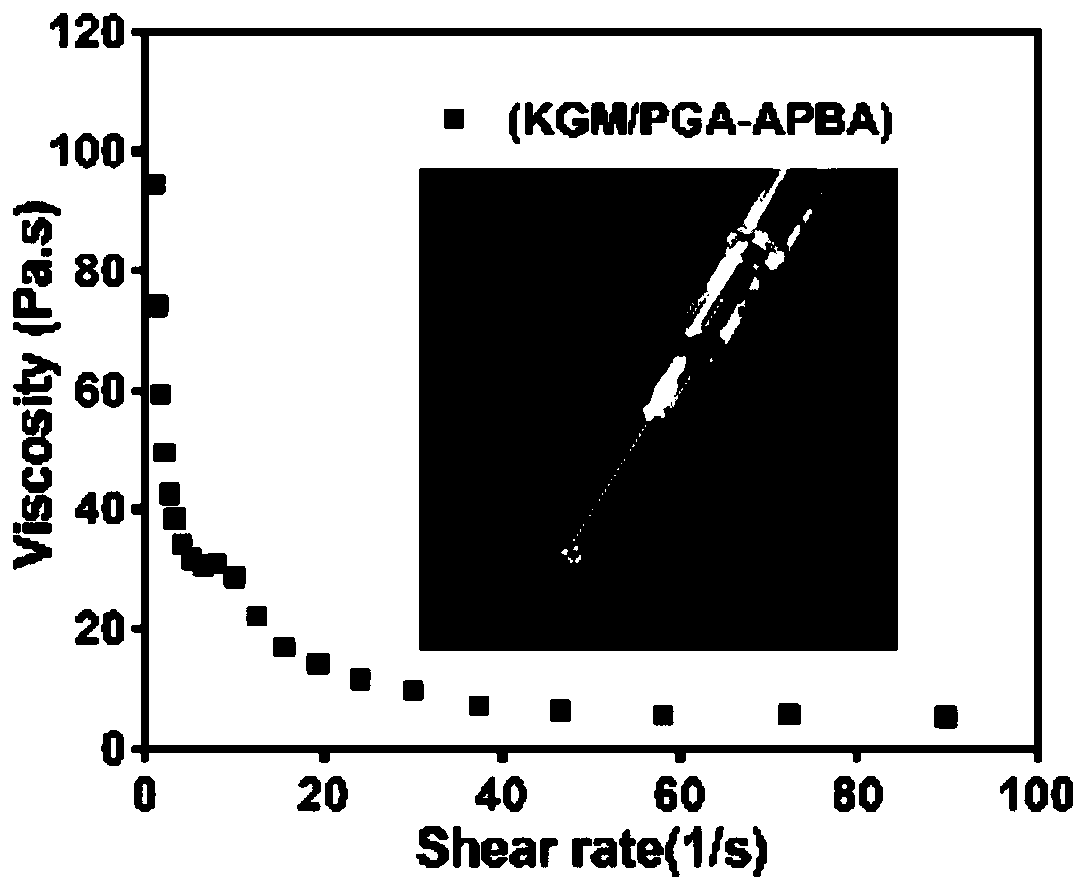
[0050] 1. Determination of viscosity of glucose-sensitive hydrogel: The apparent viscosity of glucose-sensitive hydrogel is measured by using a rheometer (TRILOS RH-x), the parameters are set to 30mm Parallel Plate, 1.0mm Gap, Speed: 5 rad / s . Each hydrogel sample was tested 3 times in parallel, and the experimental results were taken as the average of 3 times.
[0051] 2. Rheological properties of gluc...
Embodiment 3
[0055] Example 3 Glucose-sensitive insulin-loaded hydrogel preparation
[0056] Precisely weigh a certain amount of konjac glucomannan to make a 1.5% solution, add a certain amount of insulin powder, adjust the pH to 5.0, stir well, and the insulin dissolves under alkaline conditions. Then add thiol / phenylboronic acid group modified polymer, the final concentration is 2%, stir and swell to form glucose-sensitive insulin-loaded hydrogel. Its apparent viscosity is 45±3.5 Pa.s, and its elastic modulus (G′) is around 80±5.6 Pa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com