Fully human monoclonal neutralizing antibody for resisting novel coronavirus and application of fully human monoclonal neutralizing antibody
A monoclonal, antibody technology, used in antiviral agents, antiviral immunoglobulins, applications, etc., can solve the problems of infection risks of other diseases, inability to be widely used, and large differences between batches.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Screening and preparation of fully human anti-SARS-CoV-2 monoclonal antibody
[0085] 1.1 Labeling of protein probes
[0086] Coupling the required SARS-CoV-2-related specific probes to fluorescent dyes, the process is as follows:
[0087] 1.1.1 Prepare the reaction system according to the instructions of the AviTag Fusion Protein Biotinylation Kit (BirA500, Avidity). Incubate at 30°C for 30 min.
[0088] 1.1.2 Use a 30 kDa ultrafiltration tube to exchange the reaction product with 1× PBS (pH 7.4) to remove excess biotin.
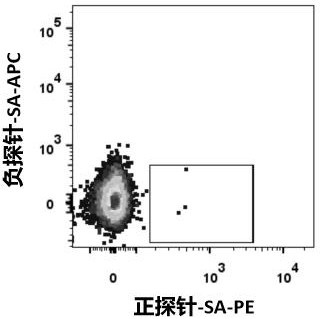
[0089] 1.1.3 Gradually add 1 / 5 molar equivalent of streptavidin-phycoerythrin (SA-PE) or streptavidin-allophycocyanin (SA-APC) to the biotin label at intervals of 20 min in the antigen-specific probe until the molar ratio of SA-PE or SA-APC:biotin-labeled antigen-specific probe reaches 1:1. Incubate with gentle shaking at 4 °C. Fluorescein-labeled probes were thus prepared: Ag(-)-SA-APC and Ag(+)-SA-PE, respectively.
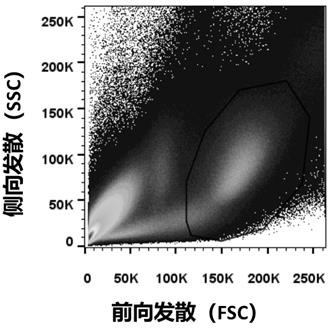
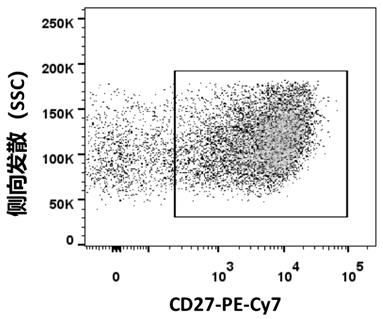
[0090] 1.2 Sepa...
Embodiment 2
[0123] Example 2 Analysis of the binding activity of YK-CoV2-01 antibody to RBD protein
[0124] Antibody-antigen binding activity analysis using Gator label-free biomolecular analyzer
[0125] 2.1. Expression of RBD-chimera using GNTI cells. After being purified by nickel column and molecular sieve sequentially, the RBD chimera was biotinylated according to the steps indicated in the manual of DSB-X™ Biotin Protein Labeling Kit (Invitrogen™).
[0126] 2.2 The biotinylated RBD chimera was diluted 600 times, and 3 μL of the RBD chimera was added to 2000ul HBSEP buffer and mixed. The diluted RBD was loaded into a 96-well sample plate at 200 μL per well.
[0127] 2.3 Use HRV3C enzyme to cut the antibody to be detected into Fab fragments, and use affinity chromatography and molecular sieve to purify the obtained antibody Fab. After diluting the antibody Fab by 1000 times, continue to perform serial dilution according to 1:3, a total of 4 dilutions (36.2nM, 12.1 nM, 4.02 nM and ...
Embodiment 3
[0132] Example 3 Analysis of YK-CoV2-01 Antibody Competitively Blocking the Binding Ability of SARS-CoV2 RBD and Receptor ACE2
[0133] Analysis of Antibody Blocking Binding Ability of SARS-CoV-2 Viral RBD Chimera and Receptor ACE2 Using Gator Non-Labeled Biomolecular Analyzer
[0134] 3.1 Using Expi-CHO cells to express the receptor ACE2. After purification by nickel column and molecular sieve in sequence, ACE2 was biotinylated according to the steps indicated in the manual of DSB-X™ Biotin Protein Labeling Kit (Invitrogen™). The biotinylated ACE2 receptor was diluted to 40ug / ml, and the molar concentration was 570nM.
[0135] 3.2 Dilute RBD to 30 ug / ml, molar concentration 1000 nM. The antibody was diluted to 0.2mg / ml and mixed 1:1 with the RBD chimera. The molar quantity of the antibody in the prepared mixture is greater than the molar quantity of the receptor ACE2.
[0136] 3.3 During the detection process, each experiment is first equilibrated in the HBSEP buffer of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com