Patents
Literature
41 results about "Nipah virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nipah Virus (NiV) Nipah virus (NiV) is a member of the family Paramyxoviridae, genus Henipavirus. NiV was initially isolated and identified in 1999 during an outbreak of encephalitis and respiratory illness among pig farmers and people with close contact with pigs in Malaysia and Singapore.
Human Monoclonal Antibodies Against Hendra and Nipah Viruses
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
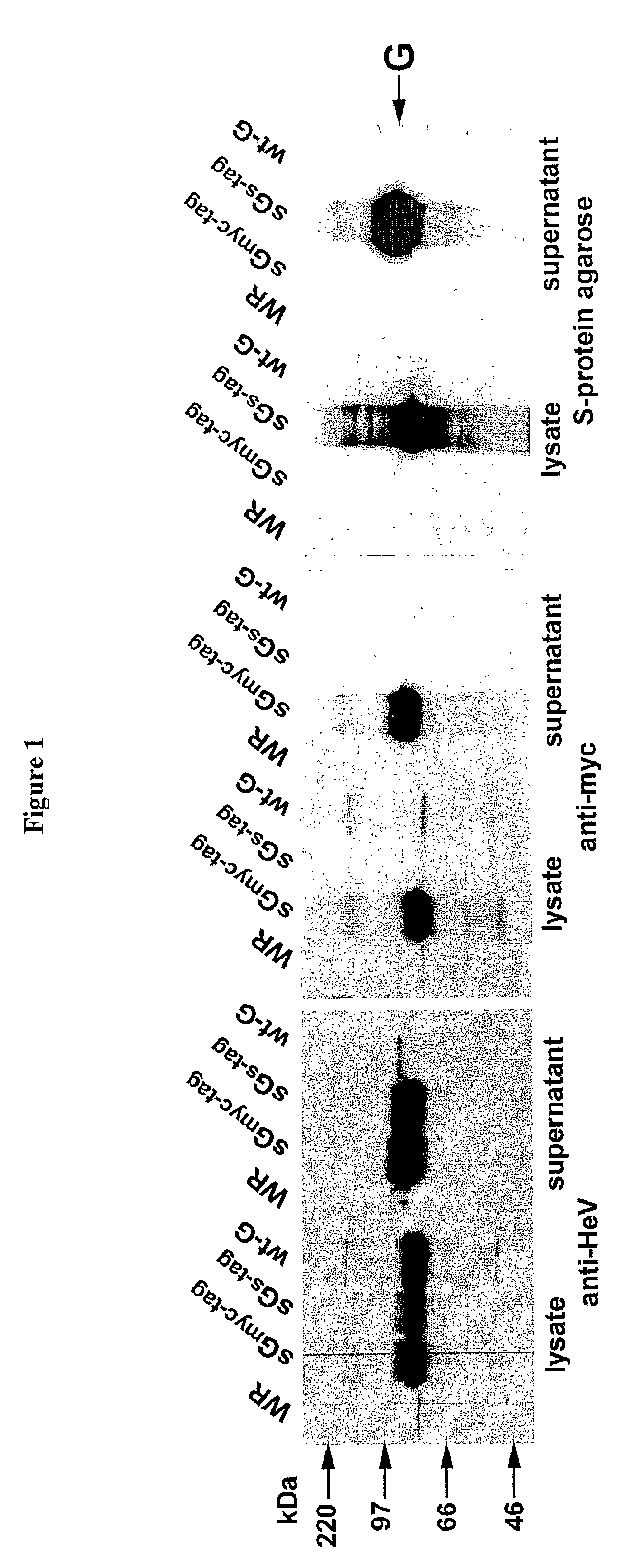
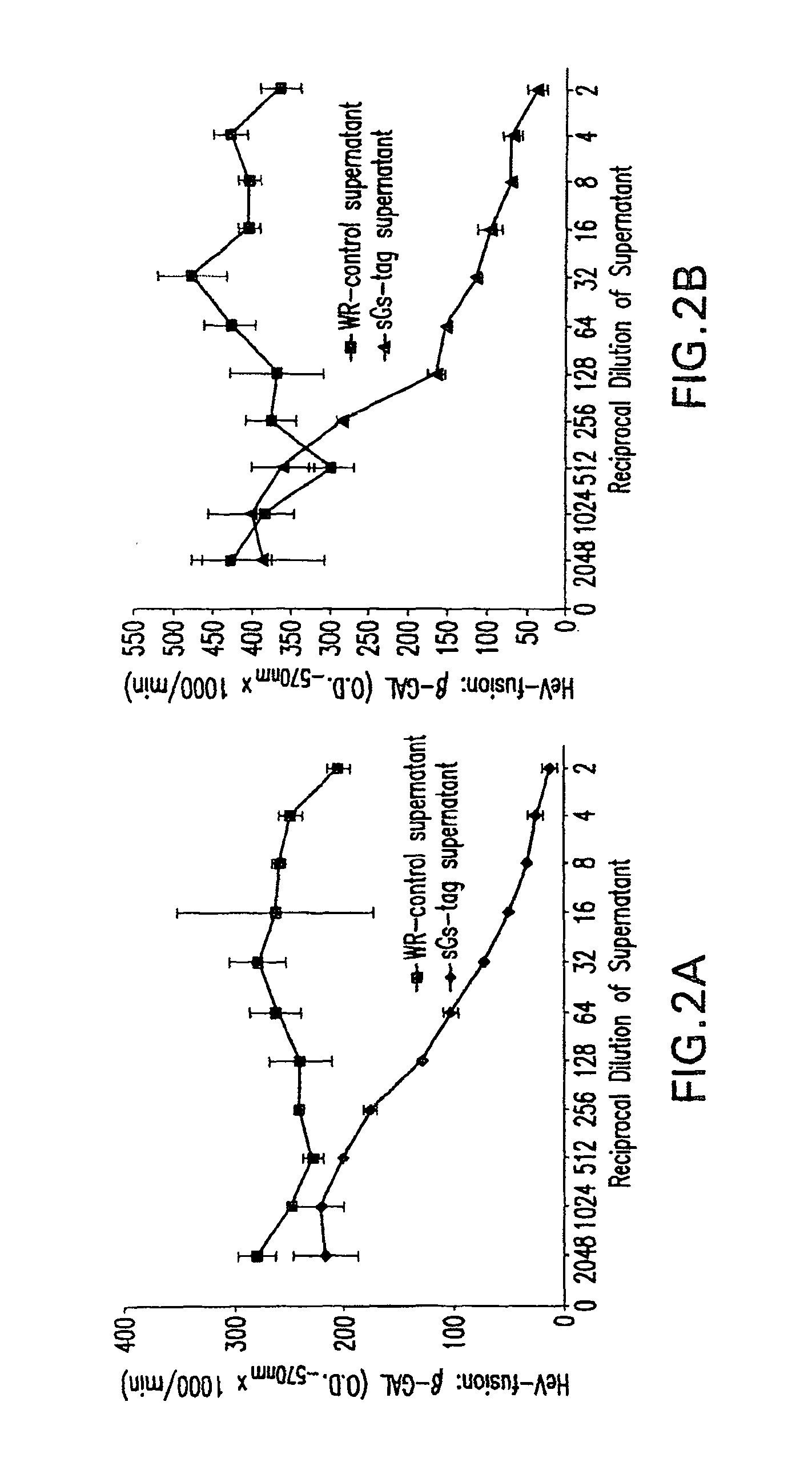
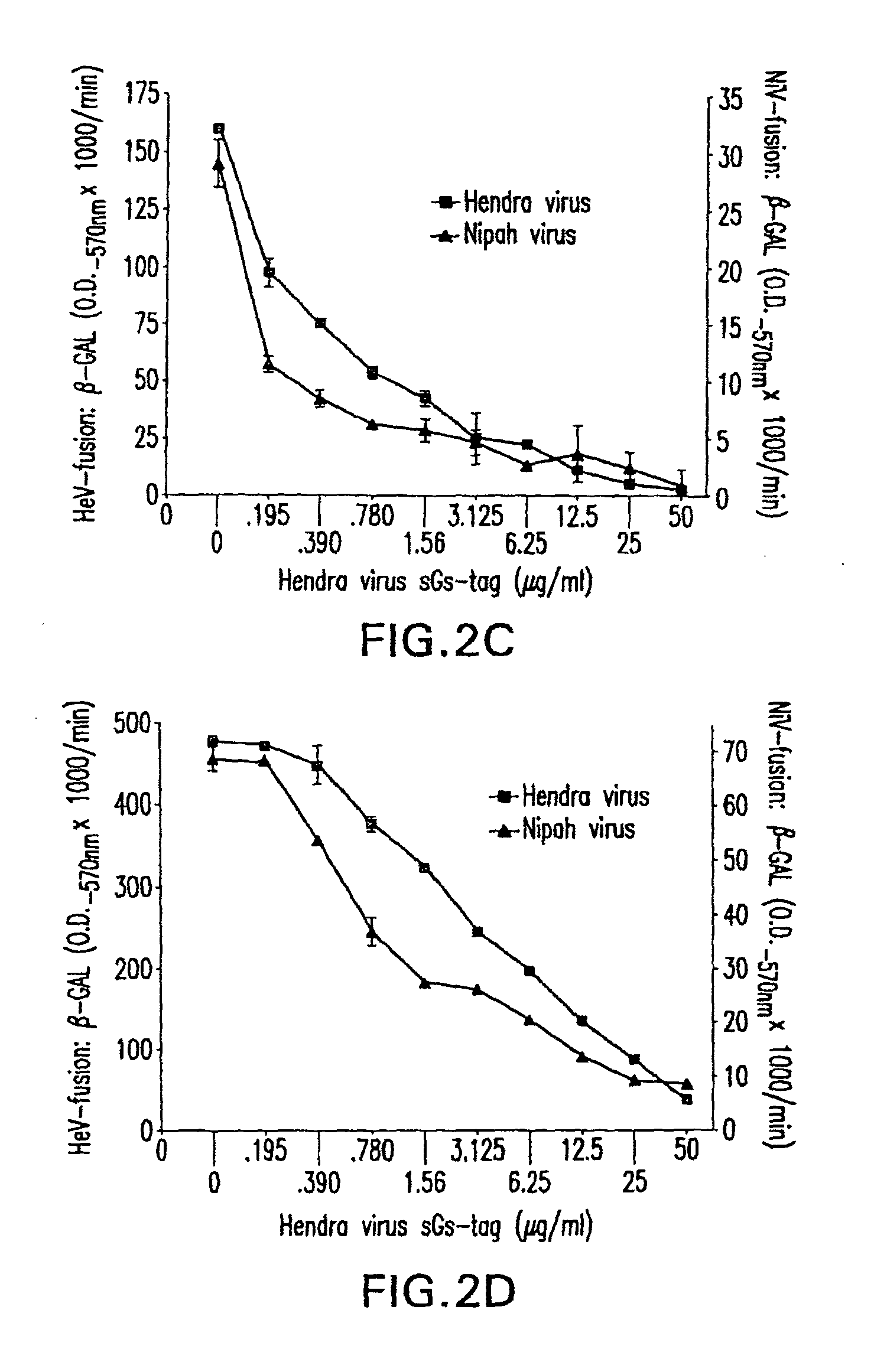
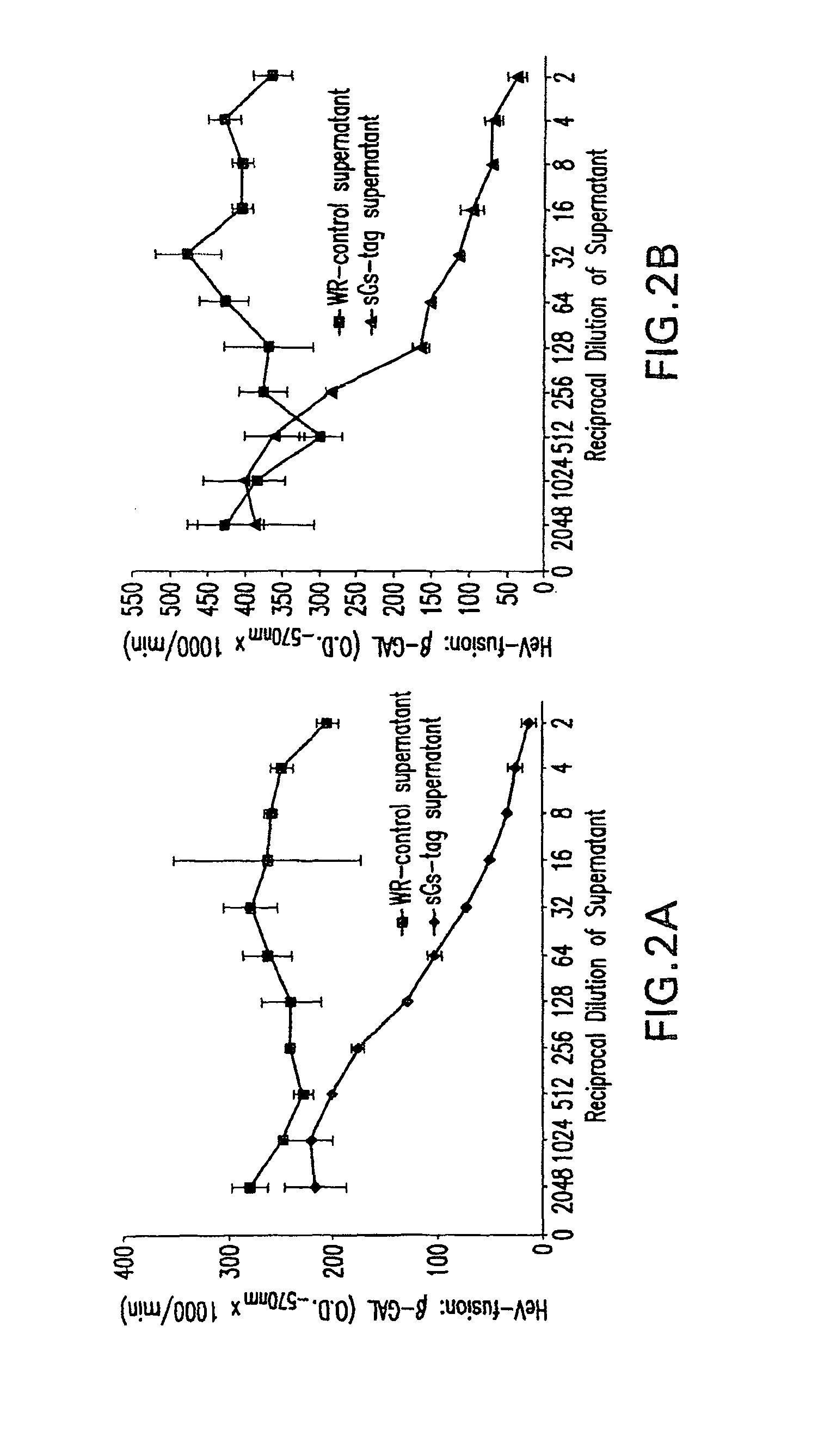
Soluble Forms of Hendra and Nipah Virus G Glycoprotein
ActiveUS20090041772A1Improve stabilityImproving immunogenicitySsRNA viruses negative-sensePeptide/protein ingredientsTherapeutic antibodyNeutralizing antibody
This invention relates to soluble forms of G glycoprotein from Hendra and Nipah virus. In particular, this invention relates to compositions comprising soluble forms of G glycoprotein from Hendra and Nipah virus and also to diagnostic and therapeutic methods using the soluble forms of G glycoprotein from Hendra and Nipah virus. Further, the invention relates to therapeutic antibodies including neutralizing antibodies, and vaccines for the prevention and treatment of infection by Hendra and Nipah viruses.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Nipah virus vaccines
ActiveUS20070031455A1Easy to storeSsRNA viruses negative-senseSugar derivativesViral glycoproteinViral Vaccine
The present invention relates to recombinant anti-Nipah virus vaccines and the administration of such vaccines to animals, advantageously pigs. Advantageously, the anti-Nipah virus vaccine may comprise a recombinant avipox virus containing a Nipah virus glycoprotein gene. The invention encompasses methods of vaccinating animals, advantageously pigs, by administration of anti-Nipah virus vaccines that may comprise a recombinant avipox virus that may contain a Nipah virus glycoprotein gene.
Owner:MERIAL INC
Human monoclonal antibodies against Hendra and Nipah viruses
ActiveUS7988971B2In-vivo radioactive preparationsViral antigen ingredientsNipah virusBinding ability
The present invention relates to monoclonal antibodies that bind or neutralize Hendra or Nipah virus. The invention provides such antibodies, fragments of such antibodies retaining Hendra or Nipah virus-binding ability, fully human antibodies retaining Hendra or Nipah virus-binding ability, and pharmaceutical compositions including such antibodies. The invention further provides for isolated nucleic acids encoding the antibodies of the invention and host cells transformed therewith. Additionally, the invention provides for prophylactic, therapeutic, and diagnostic methods employing the antibodies and nucleic acids of the invention.
Owner:UNITED STATES OF AMERICA +1
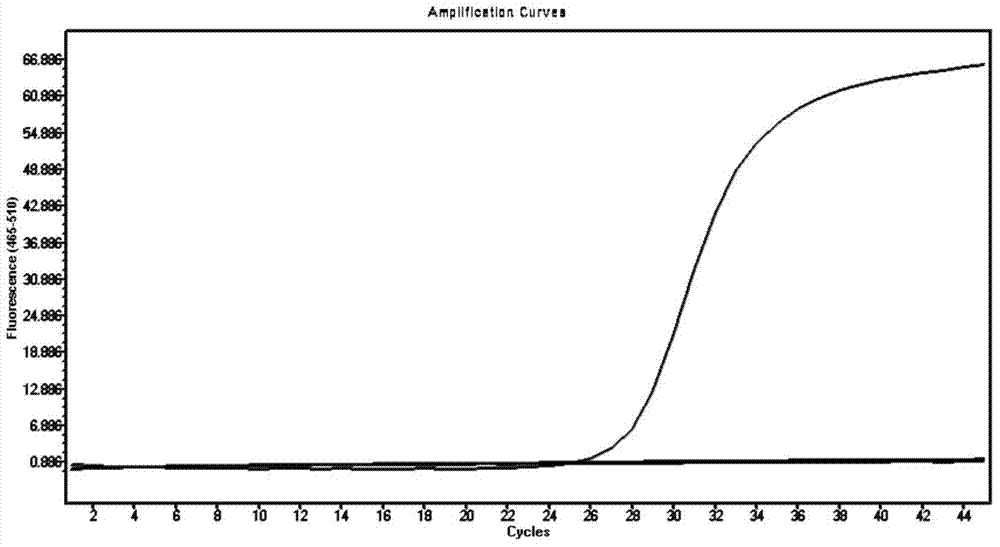
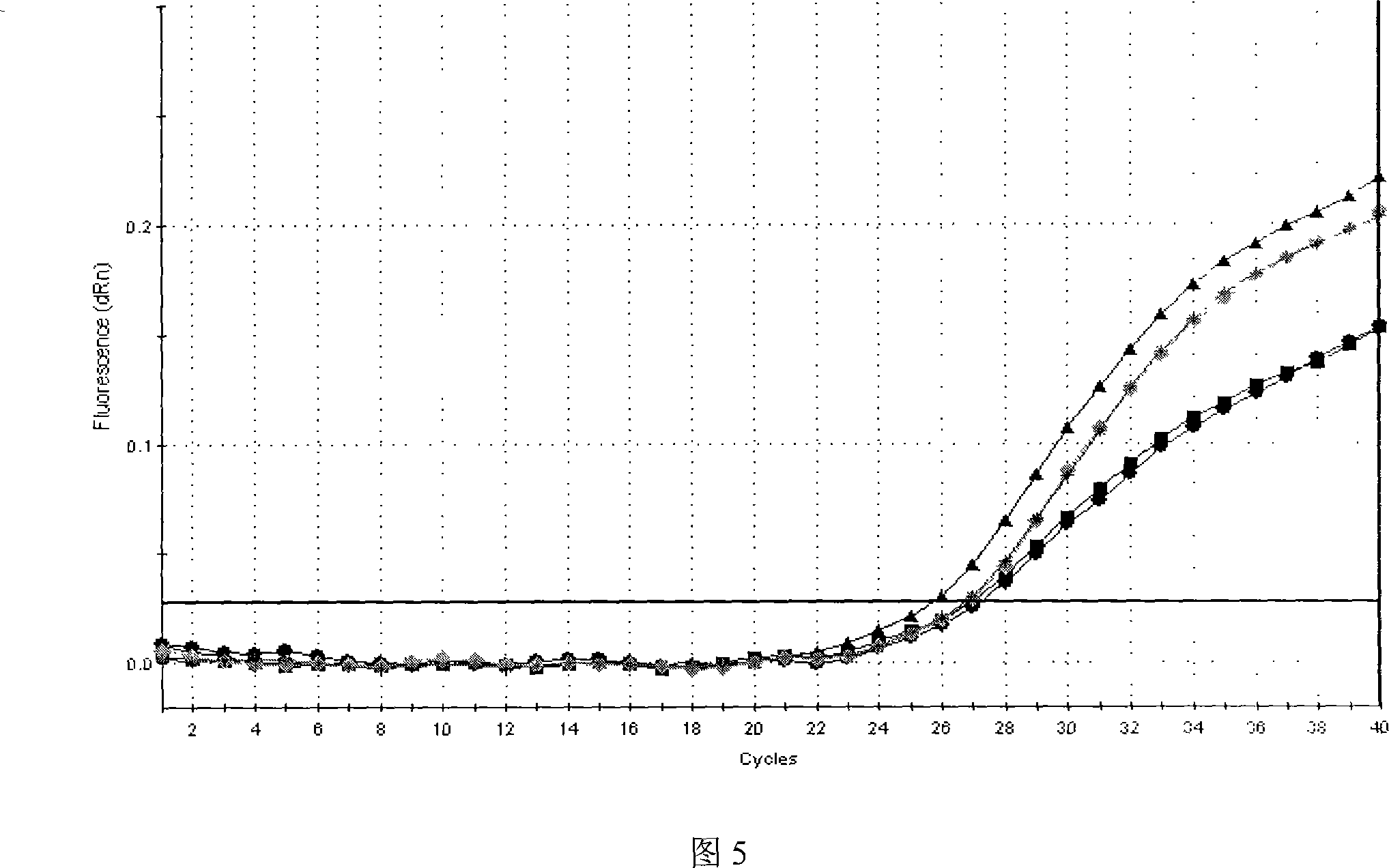
M-gene based fluorescent RT-PCR (Reverse Transcription Polymerase Chain Reaction) detection method of Nipah virus
InactiveCN102559935ANo amplification reactionIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesPositive controlFluorescence
The invention discloses an M-gene based fluorescent RT-PCR (Reverse Transcription Polymerase Chain Reaction) detection method of Nipah virus, and a kit thereof. The method adopts a one-step fluorescent RT-PCR method to detect the Nipah virus in porcine samples, is simple, convenient and fast to operate, and has higher specificity and sensitivity. According to the method, false viruses without infectivity are adopted by the positive control sample, the property is stable, false viruses do not need to be cultured, and the laboratory biosafety is good.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Nipah virus vaccines
Owner:MERIAL INC
Nipah virus envelope pseudotyped lentiviruses and methods of their use
ActiveUS9486539B2Increasing and decreasing infectivitySsRNA viruses negative-senseNon-active genetic ingredientsGlycoproteinNipah virus
The present invention relates to lentiviral particles which have been pseudotyped with Nipah virus (NiV) fusion (F) and attachment (G) glycoproteins (NiVpp-F / G). Additionally, the present invention relates to truncated NiV-F glycoproteins useful in producing such NiVpp lentiviral particles, as well as to additional variant peptides which enhance activity. Further, the present invention relates to methods of using such lentiviral particles or sequences, for example in the treatment of cancer or CNS disorders.
Owner:RGT UNIV OF CALIFORNIA
Nipah Virus Vaccines
ActiveUS20100278862A1SsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinPox viruses
The present invention relates to recombinant anti-Nipah virus vaccines and the administration of such vaccines to animals, advantageously pigs. Advantageously, the anti-Nipah virus vaccine may comprise a recombinant avipox virus containing a Nipah virus glycoprotein gene. The invention encompasses methods of vaccinating animals, advantageously pigs, by administration of anti-Nipah virus vaccines that may comprise a recombinant avipox virus that may contain a Nipah virus glycoprotein gene.
Owner:MERIAL INC
Antibodies against f glycoprotein of hendra and nipah viruses
The present invention relates to antibodies or antibody fragments that bind, neutralize, and / or inhibit Hendra or Nipah virus. The invention provides antibodies or antibody fragments that selectively bind to the F glycoprotein of Hendra or Nipah virus, and pharmaceutical compositions including such antibodies and / or fragments. The invention further provides polynucleotides encoding the antibodies and fragments of the invention and host cells transformed therewith. Additionally, the invention discloses prophylactic, therapeutic, and diagnostic methods employing the antibodies, fragments, polynucleotides, and / or compositions of the invention.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Soluble Forms of Hendra and Nipah Virus F Glycoprotein and Uses Thereof
ActiveUS20110223172A1Enhance the stability of the present invention enhance the immunogenicitySsRNA viruses negative-senseVirus peptidesNeutralizing antibodyGlycoprotein
This invention relates to soluble forms of F glycoprotein from Hendra and Nipah virus and to compositions comprising soluble forms of F glycoprotein from Hendra and Nipah virus. This invention further relates to soluble oligomers of F glycoprotein from Hendra and Nipah virus. This invention also relates to nucleic acids encoding soluble forms of F glycoprotein from Hendra and Nipah virus. This invention also relates to diagnostic and therapeutic methods using the soluble forms of F glycoprotein from Hendra and Nipah virus. Further, this invention relates to antibodies, including neutralizing antibodies, and to vaccines for the prevention, diagnosis and treatment of infection by Hendra and Nipah viruses.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Nipah virus envelope pseudotyped lentiviruses and methods of their use
ActiveUS20150050242A1Increasing and decreasing infectivitySsRNA viruses negative-senseBiocideGlycoproteinVirology
The present invention relates to lentiviral particles which have been pseudotyped with Nipah virus (NiV) fusion (F) and attachment (G) glycoproteins (NiVpp-F / G). Additionally, the present invention relates to truncated NiV-F glycoproteins useful in producing such NiVpp lentiviral particles, as well as to additional variant peptides which enhance activity. Further, the present invention relates to methods of using such lentiviral particles or sequences, for example in the treatment of cancer or CNS disorders.
Owner:RGT UNIV OF CALIFORNIA
Hendra and nipah virus g glycoprotein immunogenic compositions
This invention relates to Hendra and Nipah immunogenic compositions and methods of use. The invention also relates to methods of distinguishing subjects vaccinated with the immunogenic compositions of the invention from those infected with Hendra and / or Nipah virus.
Owner:ZOETIS SERVICE LLC
Hendra and nipah virus g glycoprotein immunogenic compositions
InactiveUS20160331829A1Efficient productionReduce reproductionSsRNA viruses negative-senseViral antigen ingredientsImmunogenicityGlycoprotein
This invention relates to Hendra and Nipah immunogenic compositions and methods of use. The invention also relates to methods of distinguishing subjects vaccinated with the immunogenic compositions of the invention from those infected with Hendra and / or Nipah virus.
Owner:ZOETIS SERVICE LLC
Soluble Forms of Hendra and Nipah Virus G Glycoprotein
ActiveUS20130171131A1Improve stabilityImproving immunogenicitySsRNA viruses negative-senseAntibody mimetics/scaffoldsTherapeutic antibodyHendra Virus
This invention relates to soluble forms of G glycoprotein from Hendra and Nipah virus. In particular, this invention relates to compositions comprising soluble forms of G glycoprotein from Hendra and Nipah virus and also to diagnostic and therapeutic methods using the soluble forms of G glycoprotein from Hendra and Nipah virus. Further, the invention relates to therapeutic antibodies including neutralizing antibodies, and vaccines for the prevention and treatment of infection by Hendra and Nipah viruses.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Broad antiviral therapy with membrane modifying oxysterols
InactiveUS20150133420A1Organic active ingredientsMicrobiological testing/measurementOxysterolIn vivo
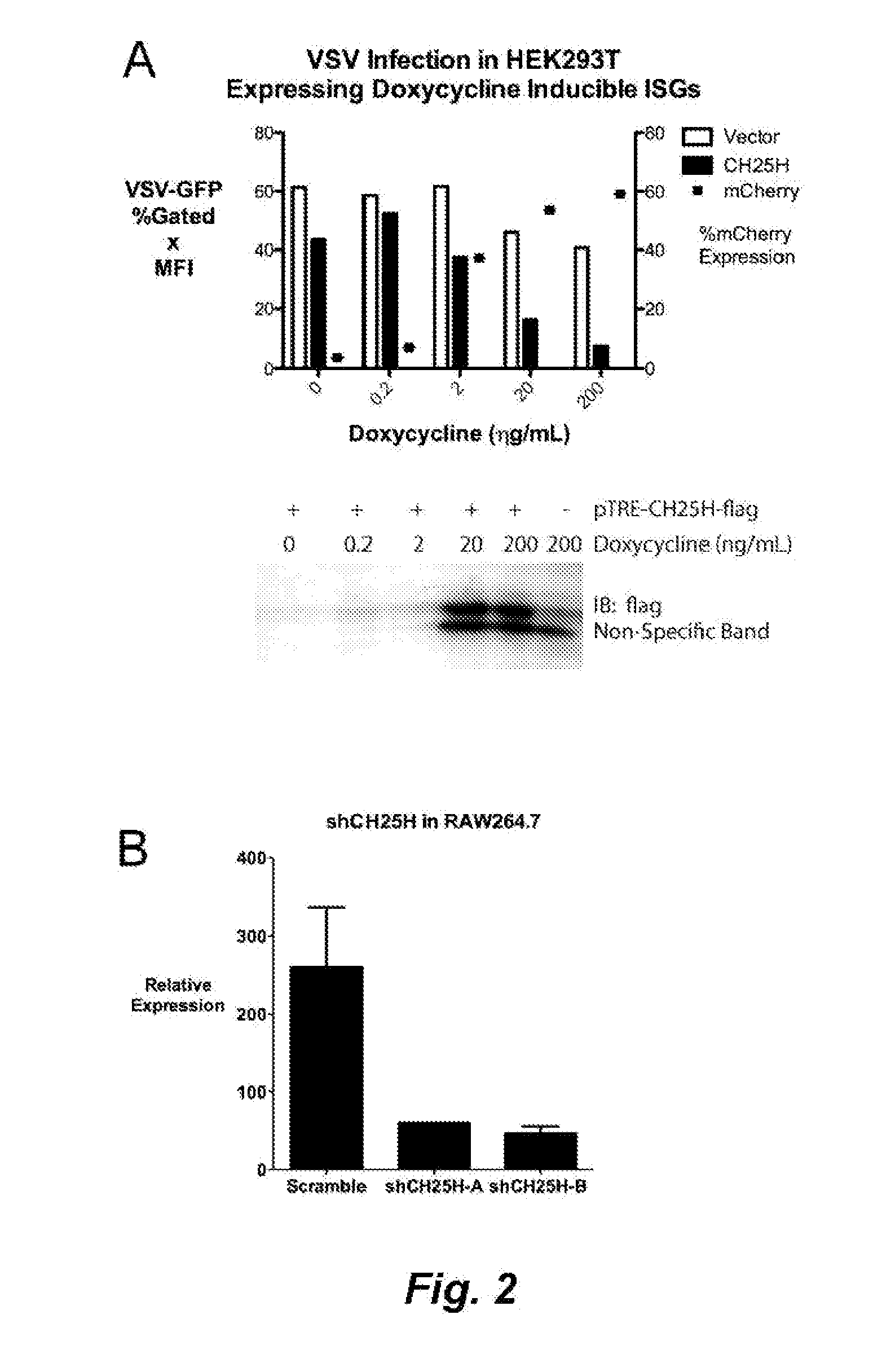
This invention relates, e.g., to a method for inhibiting the growth and / or proliferation and / or infectivity of a virus in a cell, such as a mammalian cell (e.g. for inhibiting entry of the virus into the cell), comprising administering, or causing to be administered, to the cell, 25-hydroxycholesterol (25HC) in an amount sufficient to inhibit the growth and / or proliferation and / or infectivity of the virus in the cell. The method can be carried out in vivo or in vitro. Among the viruses that can be inhibited are, e.g., VSV, HSV, MHV68, HCV, HIV, EBOV, RVFV, RSSEV and Nipah virus. In one embodiment of the invention, the 25HC is administered topically, e.g. to a mucosal surface.
Owner:RGT UNIV OF CALIFORNIA
Duplex fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for Nipah virus and porcine reproductive and respiratory syndrome virus (PRRSV) as well as preparation method and application of duplex fluorescent RT-PCR detection reagent
InactiveCN104745578AMicrobiological testing/measurementDNA/RNA fragmentationPositive controlFluorescence
The invention discloses a duplex fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for a Nipah virus and porcine reproductive and respiratory syndrome virus (PRRSV) as well as a preparation method and application of the duplex fluorescent RT-PCR detection reagent. Two sets of specific primers and Taqman probes which are respectively used for an M gene of the nipah virus and an NSP2 gene of the PRRSV as well as positive control are designed and synthesized; a duplex fluorescent RT-PCR detection system with the advantages of quickness, simplicity, convenience, high specificity and high sensitivity is established by using the two sets of primers and probes; the duplex fluorescent RT-PCR detection system can be used for simultaneously detecting nucleic acids of the Nipah virus and the PRRSV quickly, accurately, specifically, safely, simply and conveniently from a detected sample within 3 to 4 hours, and simultaneously detecting nucleic acids of trace Nipah virus and the PRRSV from a pig and a correlated sample of the pig.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Gene chip for detecting 6 viruses of zoonosis-borne diseases and application thereof
InactiveCN102031311AStable and specific universal detectionStable and specific realization of universal detectionNucleotide librariesMicrobiological testing/measurementHepacivirusNipah virus
The invention discloses a gene chip for viruses of zoonosis-borne diseases and application thereof. The gene chip for the viruses of the zoonosis-borne diseases is a gene chip for detecting at least one virus in six viruses of hepatitis E virus, Nipah virus, rabies virus, Rift Valley fever virus, foot and mouth disease virus and Vesicular stomatitis virus, and is fixed with a hepatitis E virus detection probe, a Nipah virus detection probe, a rabies virus detection probe, a Rift Valley fever virus detection probe, a foot and mouth disease virus detection probe and a Vesicular stomatitis virus detection probe. The invention also discloses a method for detecting the Rift Valley fever virus, the Nipah virus, the foot and mouth disease virus, the Vesicular stomatitis virus, the hepatitis E virus and / or the rabies virus. The invention can stably and specifically implement generic detection of the six viruses of the zoonosis-borne diseases, has the advantages of simpleness, convenience, quickness, good specificity and high sensitivity, and can be used for detecting clinical samples.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Hendra and nipah virus g glycoprotein immunogenic compositions
InactiveUS20150050305A1Efficient productionReduce reproductionSsRNA viruses negative-senseViral antigen ingredientsImmunogenicityGlycoprotein
Immunogenic compositions directed against Hendra and / or Nipah viruses, and methods of its use, are provided. In addition, methods of distinguishing subjects vaccinated with the immunogenic compositions of the invention from those infected with Hendra and / or Nipah virus are provided.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +2
Antibodies against F glycoprotein of Hendra and Nipah viruses
The present invention relates to antibodies or antibody fragments that bind, neutralize, and / or inhibit Hendra or Nipah virus. The invention provides antibodies or antibody fragments that selectively bind to the F glycoprotein of Hendra or Nipah virus, and pharmaceutical compositions including such antibodies and / or fragments. The invention further provides polynucleotides encoding the antibodies and fragments of the invention and host cells transformed therewith. Additionally, the invention discloses prophylactic, therapeutic, and diagnostic methods employing the antibodies, fragments, polynucleotides, and / or compositions of the invention.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Soluble Forms of Hendra and Nipah Virus G Glycoprotein
ActiveUS20130171132A1Improve stabilityImproving immunogenicitySsRNA viruses negative-senseAntibody mimetics/scaffoldsTherapeutic antibodyNeutralizing antibody
This invention relates to soluble forms of G glycoprotein from Hendra and Nipah virus. In particular, this invention relates to compositions comprising soluble forms of G glycoprotein from Hendra and Nipah virus and also to diagnostic and therapeutic methods using the soluble forms of G glycoprotein from Hendra and Nipah virus. Further, the invention relates to therapeutic antibodies including neutralizing antibodies, and vaccines for the prevention and treatment of infection by Hendra and Nipah viruses.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
In-vitro Nipah virus detection reagent and in-vitro Nipah virus detection method
InactiveCN105567870ASimple and fast operationMicrobiological testing/measurementMicroorganism based processesForward primerFluorescence
The invention provides an in-vitro Nipah virus detection reagent. The in-vitro Nipah virus detection reagent comprises specific primers and a probe. A sequence of a forward primer NIF is shown as 5'-GCAAGAGAGTAATGTTCAGGCTAGAG-3'; a sequence of a reverse primer NIR is shown as 5'-CTGTTCTATAGGTTCTTCCCCTTCAT-3'; a sequence of the probe NIP is shown as 5'FAM-TGCAGGAGGTGTGCTCATTGGAGG-TAMRA3'. The invention further provides an in-vitro fluorescent PCR (polymerase chain reaction) Nipah virus detection method which adopts the in-vitro Nipah virus detection reagent to quickly and accurately detect Nipah viruses. The in-vitro fluorescent PCR Nipah virus detection method has the advantages of simplicity and convenience in operation, high efficiency, quickness and specificity; due to establishment of the method, sample detection can be finished only by about one hour, and a gap of domestic Nipah virus detection is filled.
Owner:浙江国际旅行卫生保健中心
Encephalitis-related virus detection kit and application thereof
InactiveCN105603128AReduced risk of cross-contaminationLow costMicrobiological testing/measurementDNA/RNA fragmentationJapanese encephalitisEastern equine encephalitis virus
The invention discloses an encephalitis-related virus detection kit and application thereof. The kit is used on the basis of the principles of multiplex PCR (polymerase chain reaction) combined nucleic acid intrusive reaction and nano gold color development. The kit comprises a combination of five types of primers and probes, and can be used for simultaneously detecting eastern equine encephalitis virus, western equine encephalitis virus, epidemic encephalitis b virus, West Nile virus and Nipah virus. When being used for detecting the five viruses, the kit has the advantages of high specificity and high sensitivity, and does not need any expensive apparatus; the detection result can be observed by naked eyes; and thus, the kit can be applied to the basic level.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Fluorescent RT-PCR detection reagent of nipah virus M gene as well as preparation method and application of fluorescent RT-PCR detection reagent
InactiveCN104774972AMicrobiological testing/measurementMicroorganism based processesPositive controlFluorescent pcr
The invention discloses a fluorescent RT-PCR detection reagent of a nipah virus M gene as well as a preparation method and an application of the fluorescent RT-PCR detection reagent. A set of specific primers, a Taqman probe and a positive control are designed and synthesized, and the primers and the probe are used for establishing a fluorescent RT-PCR detection system which is quick, simple and convenient and is strong in specificity and high in sensitivity, so that the nipah virus M gene can be detected quickly, accurately, specifically, safely, simply and conveniently from a detected sample in 3-4 hours, and the fluorescent RT-PCR detection reagent can be used for detecting micro nipah virus M gene from domestic pigs and in related samples.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
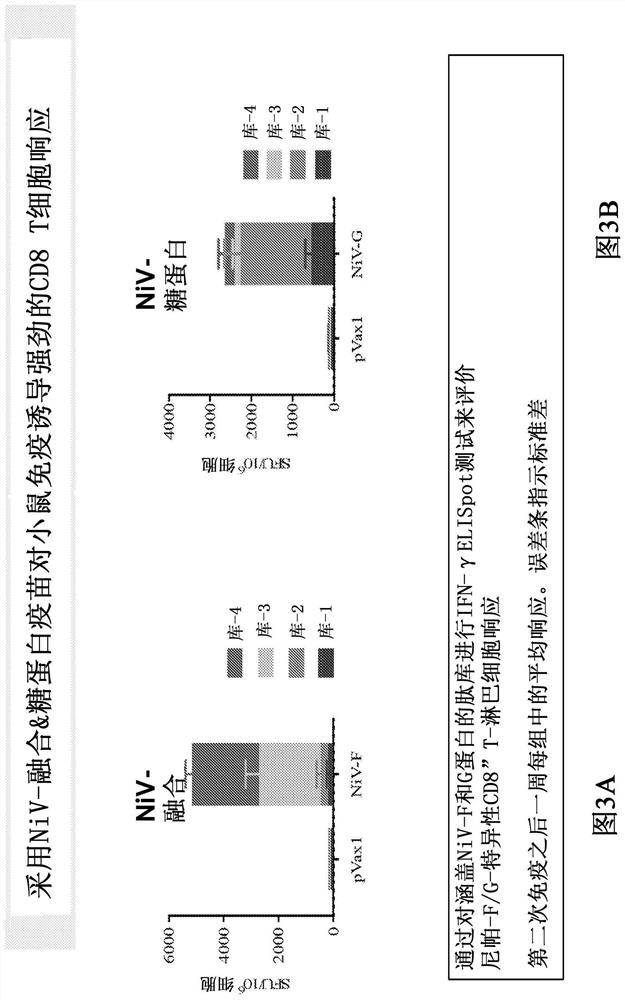
Vaccines against nipah virus, and methods of using same
An aspect of the present invention is related to nucleic acid constructs capable of expressing at least one Nipah virus (NiV) antigen that elicits an immune response in a mammal against NiV virus, and methods of use thereof.
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY
Duplex fluorescent RT-PCR detection reagent for Nipah virus and hog cholera virus as well as preparation method and application thereof
InactiveCN104762404ASafeImprove economyMicrobiological testing/measurementDNA/RNA fragmentationPositive controlFluorescence
The invention discloses a duplex fluorescent RT-PCR detection reagent for nipah virus and hog cholera virus as well as a preparation method and an application thereof. Two sets of specific primers for a nipah virus M gene and a hog cholera virus 5' UTR gene as well as a Taqman probe and positive control are designed and synthesized respectively, and a duplex fluorescent RT-PCR detection system which is quick, simple, convenient, strong in specificity and high in sensitivity is established by utilizing the two sets of primers and the probe, so that nucleic acids of the nipah virus and the hog cholera virus can be quickly, accurately, specifically, safely, simply and conveniently detected from a detected sample within 3-4 hours, and the detection reagent can be used for simultaneously detecting trace nucleic acids of the nipah virus and the hog cholera virus in a hog and a relevant sample thereof.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Nipah virus detection kit and dedicated primer of same
ActiveCN109022621AImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationNipah virusVirology
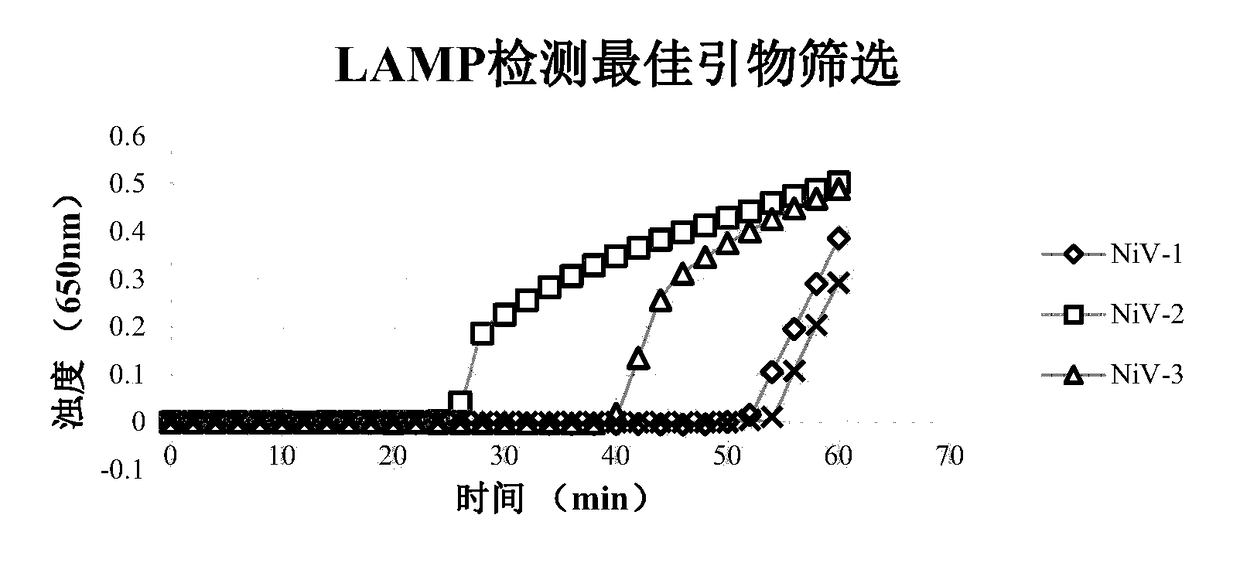
The invention discloses a Nipah virus detection kit and a dedicated primer of same, wherein the primer is used for performing RT-LAMP detection on the Nipah virus or LAMP detection on the genome cDNAof the Nipah virus. The primer is designed on the basis of specific conserved target sequences of the Nipah virus, which are represented as the SEQ ID No.1-3. The primer or detection kit in the invention can detect the Nipah virus under an isothermal condition quickly, conveniently, high-effectively, high-specifically and high-sensitively without use of complex instruments. The kit can be used forperforming the RT-LAMP detection on the genome RNA of the Nipah virus.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Affinity selection of Nipah and Hendra virus-related vaccine candidates from a complex random peptide library displayed on bacteriophage virus-like particles
ActiveUS9549976B1SsRNA viruses negative-senseViral antigen ingredientsRandom Peptide LibraryViral glycoprotein
The invention relates to virus-like particles of bacteriophage MS2 (MS2 VLPs) displaying peptide epitopes or peptide mimics of epitopes of Nipah Virus envelope glycoprotein that elicit an immune response against Nipah Virus upon vaccination of humans or animals. Affinity selection on Nipah Virus-neutralizing monoclonal antibodies using random sequence peptide libraries on MS2 VLPs selected peptides with sequence similarity to peptide sequences found within the envelope glycoprotein of Nipah itself, thus identifying the epitopes the antibodies recognize. The selected peptide sequences themselves are not necessarily identical in all respects to a sequence within Nipah Virus glycoprotein, and therefore may be referred to as epitope mimics VLPs displaying these epitope mimics can serve as vaccine. On the other hand, display of the corresponding wild-type sequence derived from Nipah Virus and corresponding to the epitope mapped by affinity selection, may also be used as a vaccine.
Owner:STC UNM
Primer and primer for Nipah virus, and one-step method real time RT-PCR detecting kit
ActiveCN101195846AQuick checkReduce false positive resultsMicrobiological testing/measurementDNA/RNA fragmentationReal-Time PCRsReference genes
The invention relates to a group of primers, a probe, and a corresponding reagent box. The invention comprises a pair of primers which can simultaneously detect Nipah virus and reference genes, and two probes for the Nipah virus and the TaqMan of the reference genes, through reducing the quantity of the primer and the probe in a PCR reaction system to be four from usual 6, the invention can realize that the Nipah virus and the reference genes perform high efficiency amplification in an identical pipe, and can realize the detection to the target genes in different species, the versatility is strong, the quantity of the primer and the probe, the cost of the laboratory reagent, and the operating steps are reduced, and the opportunity that a PCR experiment is usually required to avoid pollution is reduced. The reagent box of the invention is suitable for the scientific research or the detection of the clinical Nipah virus, in particular to the monitoring to the Nipah virus during the virosis prevention and control work.
Owner:谢鹏 +1
Duplex fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for nipah virus and swine influenza virus (SIV) as well as preparation method and application of duplex fluorescent RT-PCR detection reagent
InactiveCN104745713ASafeImprove economyMicrobiological testing/measurementDNA/RNA fragmentationPositive controlFluorescence
The invention discloses a duplex fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for Nipah virus and swine influenza virus (SIV) as well as a preparation method and application of the duplex fluorescent RT-PCR detection reagent. Two sets of specific primers and Taqman probes which are respectively used for an M gene of the Nipah virus and an M virogene of the SIV as well as positive control are designed and synthesized; a duplex fluorescent RT-PCR detection system with the advantages of quickness, simplicity, convenience, high specificity and high sensitivity is established by using the two sets of primers and probes; the duplex fluorescent RT-PCR detection system can be used for simultaneously detecting nucleic acids of the Nipah virus and the SIV quickly, accurately, specifically, safely, simply and conveniently from a detected sample within 3 to 4 hours, and simultaneously detecting nucleic acids of trace Nipah virus and the SIV from a domestic pig and a correlated sample of the domestic pig.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
A kind of anti-Nipah virus envelope glycoprotein monoclonal antibody and its application
ActiveCN110028579BCombined with effective inhibitionInhibit bindingImmunoglobulins against virusesAntiviralsDiseaseHendra Virus
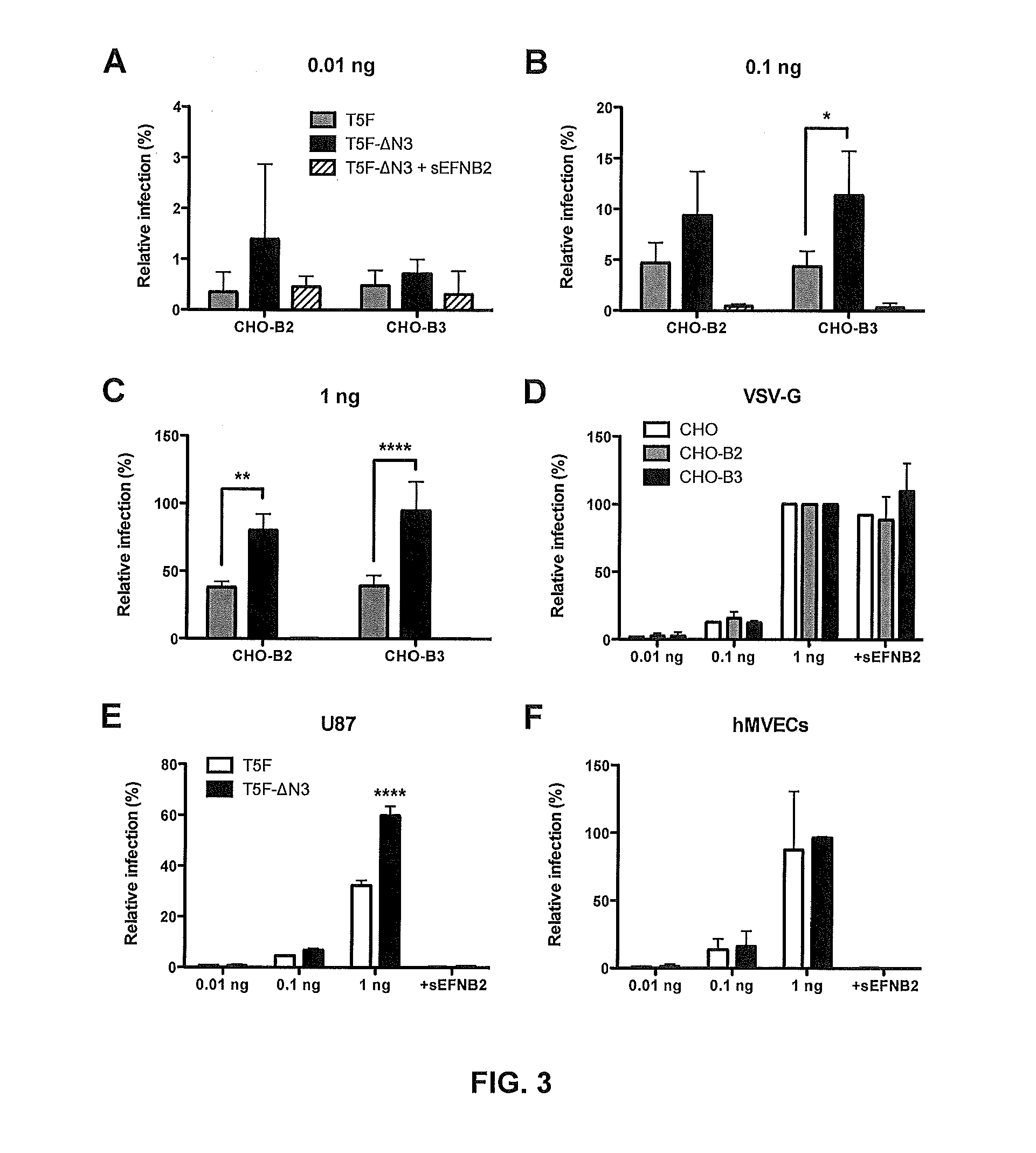
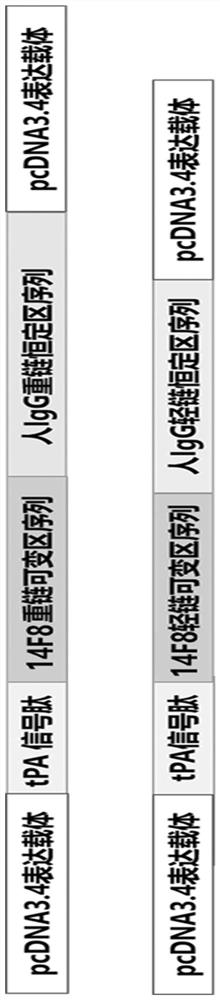
The present invention discloses a monoclonal antibody 14F8 against Nipah virus envelope glycoprotein. The antibody has a unique CDR region and has a binding titer of 0.47 ng / mL with the Nipah virus envelope glycoprotein, and when an antibody concentration is greater than 9.14 ng / mL, an ELISA OD value is greater than 2.0, indicating excellent antigen binding activity. The antibody has a binding titer of 129.66 ng / mL with Hendra virus envelope glycoprotein, and when the antibody concentration is 20,000 ng / mL, the OD value is only 0.29, indicating the 14F8 can be used for detection of the Nipah virus envelope glycoprotein and can effectively distinguish the Nipah virus envelope glycoprotein and the Hendra virus envelope glycoprotein. The present invention also discloses an application of the14F8 monoclonal antibody in preparation of drugs for treating Nipah virus diseases, the antibody can effectively inhibit binding of the Nipah virus envelope glycoprotein to a cellular receptor EFNB2,an IC50 value is 50 ng / mL, besides, neutralizing activity is enhanced with increase of the antibody concentration, and when the antibody concentration exceeds 1 [mu]g / ml, an inhibition rate tends to reach 100%, indicating a prospect of the 14F8 monoclonal antibody as a candidate therapeutic antibody for the Nipah virus diseases.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com