Patents
Literature
43 results about "Equine Encephalitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antiviral activity from medicinal mushrooms
Compounds having unique antiviral properties are prepared from medicinal mushroom mycelium, extracts and derivatives. The compositions are derived from Fomitopsis, Piptoporus, Ganoderma and blends of medicinal mushroom species and are useful in preventing and treating viruses including Orthopox viruses, influenza, avian influenza, Venezuelan Equine Encephalitis, yellow fever, West Nile, Dengue, New World and Old World arenaviruses, hantavirus, Rift Valley fever, sandfly fever, hantavirus, SARS, Rhinovirus and other viruses.
Owner:TURTLE BEAR HLDG LLC
Venezuelan equine encephalitis virus replicons with adaptive mutations in the genome and uses thereof
ActiveUS7332322B2Reduced cytopathic effectReduce productionSsRNA viruses positive-senseViral antigen ingredientsMammalViral replication
The present invention provides a Venezuelan equine encephalitis virus replicon RNA useful in the development of stable lines of mammalian, avian and insect cells in which these replicons will persistently replicate. Venezuelan equine encephalitis (VEE) virus replicons contain a number of unique adaptive mutations that make the replicons noncytopathic. The replicons remain resistant to IFN-α / β. Replicon replication leads to high-level production of heterologous proteins, which are encoded by the replicons' genome and are under the control of a viral subgenomic promoter. Also provided are methods of screening for inhibitory compounds of Venezuelan equine encephalitis virus replication and eastern equine encephalitis virus replication.
Owner:BOARD OF RGT UNIV OF TEXAS THE
Ebola virion proteins expressed from venezuelan equine encephalitis (VEE) virus replicons
InactiveUS6984504B2SsRNA viruses negative-senseSsRNA viruses positive-senseVP40Venezuelan equine encephalitis virus
Using the Ebola GP, NP, VP24, VP30, VP35 and VP40 virion proteins, a method and composition for use in inducing an immune response which is protective against infection with Ebola virus is described.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
4'-halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto
Disclosed are halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto. In certain embodiments, the disclosure relates to the treatment or prophylaxis of viral infections. Such viral infections can include tongaviridae, bunyaviridae, arenaviridae, coronaviridae, flaviviridae, picornaviridae, Eastern, Western, and Venezuelan Equine Encephalitis (EEE, WEE and VEE, respectively), Chikungunya fever (CHIK), Ebola, Influenza, RSV, and Zika virus infections.
Owner:EMORY UNIVERSITY
Live attenuated viral vaccines for Eastern Equine Encephalitis virus
InactiveUS7790181B2Improve immunityBiocideSsRNA viruses positive-senseEastern equine encephalitis virusNeutralising antibody
Live attenuated Eastern Equine Encephalitis (EEE) vaccines that outperform the PE-6 vaccine in mice aerosol challenged with >1,000×LD50. Candidates include four furin-cleavage deletion mutants and one E3 deletion mutant. Each vaccine provided protection in birds against antigenically distinct North and South American strains of EEE. The PE-6 vaccine does not provide protection against South American EEEs. Animals inoculated with each of the vaccines of the invention developed neutralizing antibodies to EEE.
Owner:ARMY UNITED STATES OF AMERICA THE AS REPRESENTED BY SEC
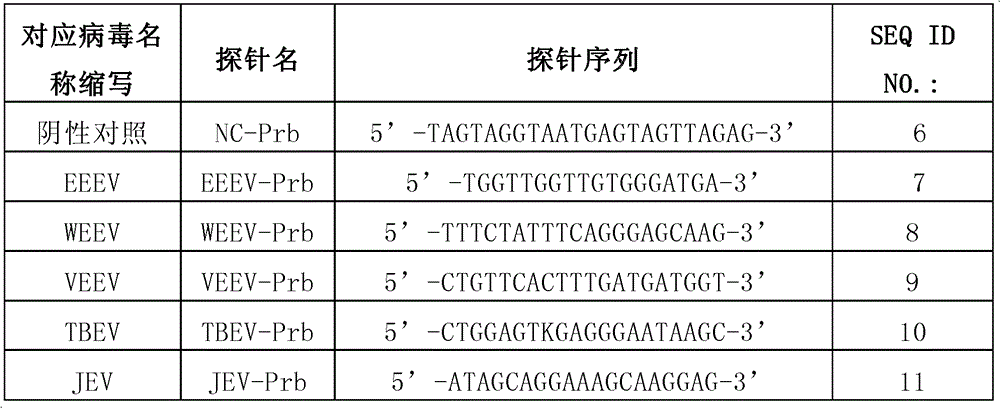
Kit for detecting eastern equine encephalitis virus and west equine encephalitis virus by real-time fluorescence quantitative RT-PCR
InactiveCN101967524AMicrobiological testing/measurementFluorescence/phosphorescenceForward primerFluorescence
The invention discloses a kit for detecting eastern equine encephalitis virus and west equine encephalitis virus by real-time fluorescence quantitative RT-PCR. The kit provided by the invention comprises a primer pair as shown in the following (1) and (2): (1) EEEV forward primer: TGTGCGTACCTCCTCATCGTT, EEEV reverse primer: GACTGGCGTGAATCTCTGCT; (2) WEEV forward primer: AGGGATACCCCCGAAGGTT, WEEV reverse primer: GTGAATAGCACACGGGTGGTT. The kit can be applied to detecting EEEV and WEEV and has good sensitivity and specificity; in addition, the lowest EEEV virus titer that the kit can detect is 0.2TCID50 / ml and the WEEV virus titer that the kit can detect is 1TCID50 / ml.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Antibody dependent enhancement of Venezuelan Equine Encephalitis vector infection
ActiveUS7651998B1Good curative effectIncrease infectivitySsRNA viruses positive-sensePeptide/protein ingredientsAntibody-dependent enhancementDendritic cell
The present invention provides compositions and methods for delivering a nucleotide sequence to a cell using an alphavirus vector that is complexed with an enhancing antibody that specifically binds to the alphavirus vector. Venezuelan Equine Encephalitis vectors are preferred. The cell may be a cell in vitro or in vivo. Alternatively, the cell may be removed from a subject, administered the alphavirus vector ex vivo and then administered to a subject. Antigen-presenting cells are preferred, with dendritic cells being more preferred. Also provided are methods of producing an immune response in a subject, e.g., for producing an immune response against an antigen associated with a pathogen or for immunotherapy of cancer of tumors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Methods of treating zika virus, mers-cov, chikungunya, venezuelan equine encephalitus, and rhinovirus in mammalian patients
InactiveUS20170157219A1Inhibition formationHydrolasesPeptide/protein ingredientsZika virusMiddle East respiratory syndrome coronavirus
Viral infections in mammals can be treated and prophylactically prevented by systemic administration of ranpirnase and three other ribonucleases that are highly homologous with it and that have activities that are highly similar to it. Experimental results against Zika virus, Middle East Respiratory Syndrome Coronavirus (“MERS-CoV”), Chikungunya virus, Venezuelan equine encephalitis, and rhinovirus-14 are disclosed.
Owner:TAMIR BIOTECH
Method and kit for detecting multiple encephalitis related viruses
ActiveCN102796827AImprove throughputAddresses issues with reduced sensitivityMicrobiological testing/measurementDNA/RNA fragmentationEastern equine encephalitis virusMicrosphere
The invention provides a method and a kit for detecting multiple encephalitis related viruses. In particular, the invention discloses a method for simultaneously detecting multiple encephalitis related viruses. The method comprises the following steps of: performing polymerase chain reaction (PCR) by using a specific primer set aiming at the encephalitis related viruses in a polymerase reaction system to obtain an amplification product; and detecting with specific probes or probe microspheres. The invention also provides the corresponding kit. The method and the kit can be used for sensitively and simply detecting and identifying multiple encephalitis related viruses comprising eastern equine encephalitis viruses, western equine encephalitis viruses, Venezuelan equine encephalitis viruses, forest encephalitis viruses and Japanese encephalitis viruses.
Owner:SHANGHAI TELLGEN LIFE SCI CO LTD
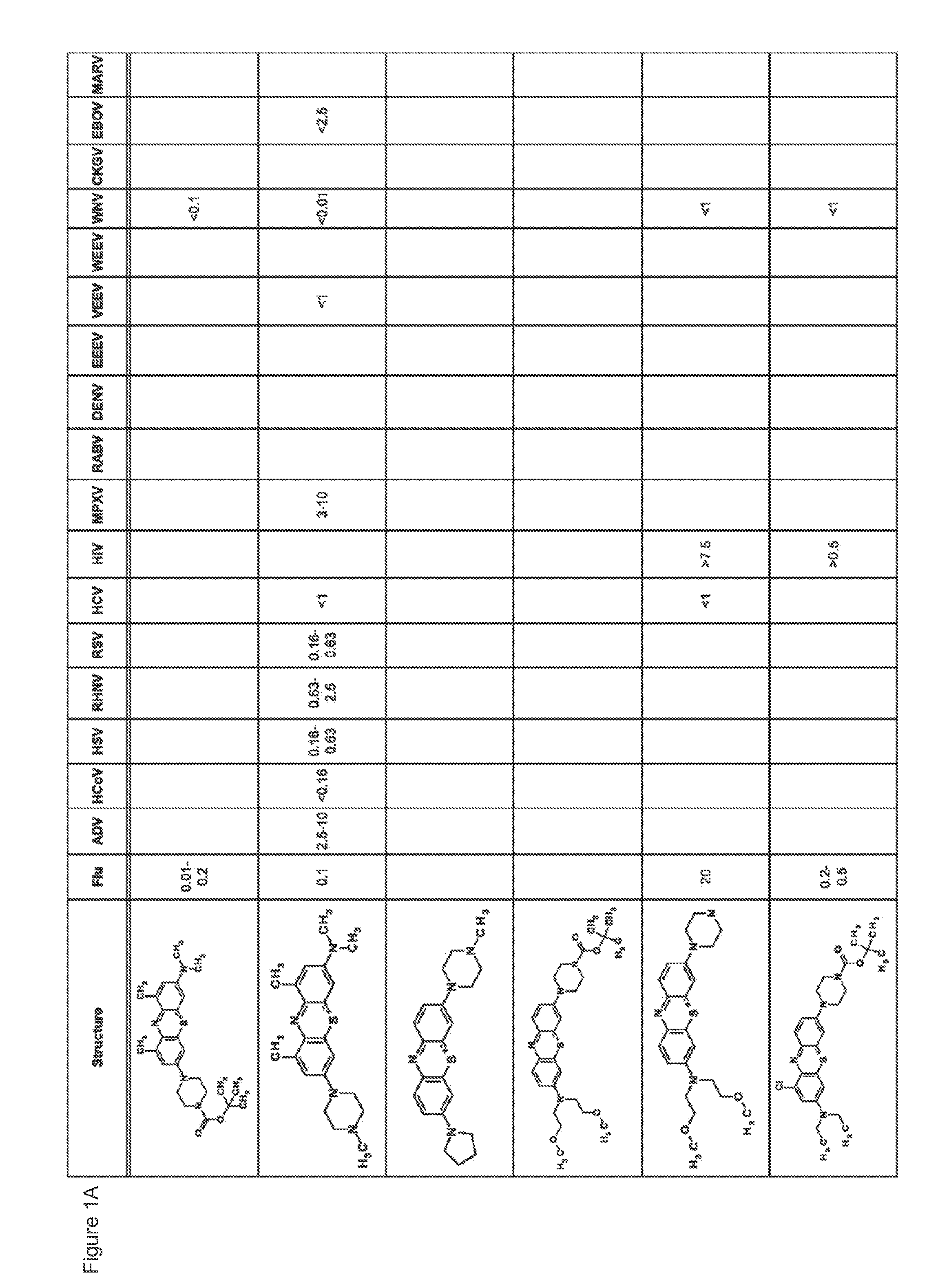
Antiviral Compounds
InactiveUS20120302556A1Easy to PlatingEliminate residual PBS.Organic chemistryAntiviralsViral infectionWest Nile virus RNA
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and Hepatitis C.
Owner:PROSETTA ANTIVIRAL
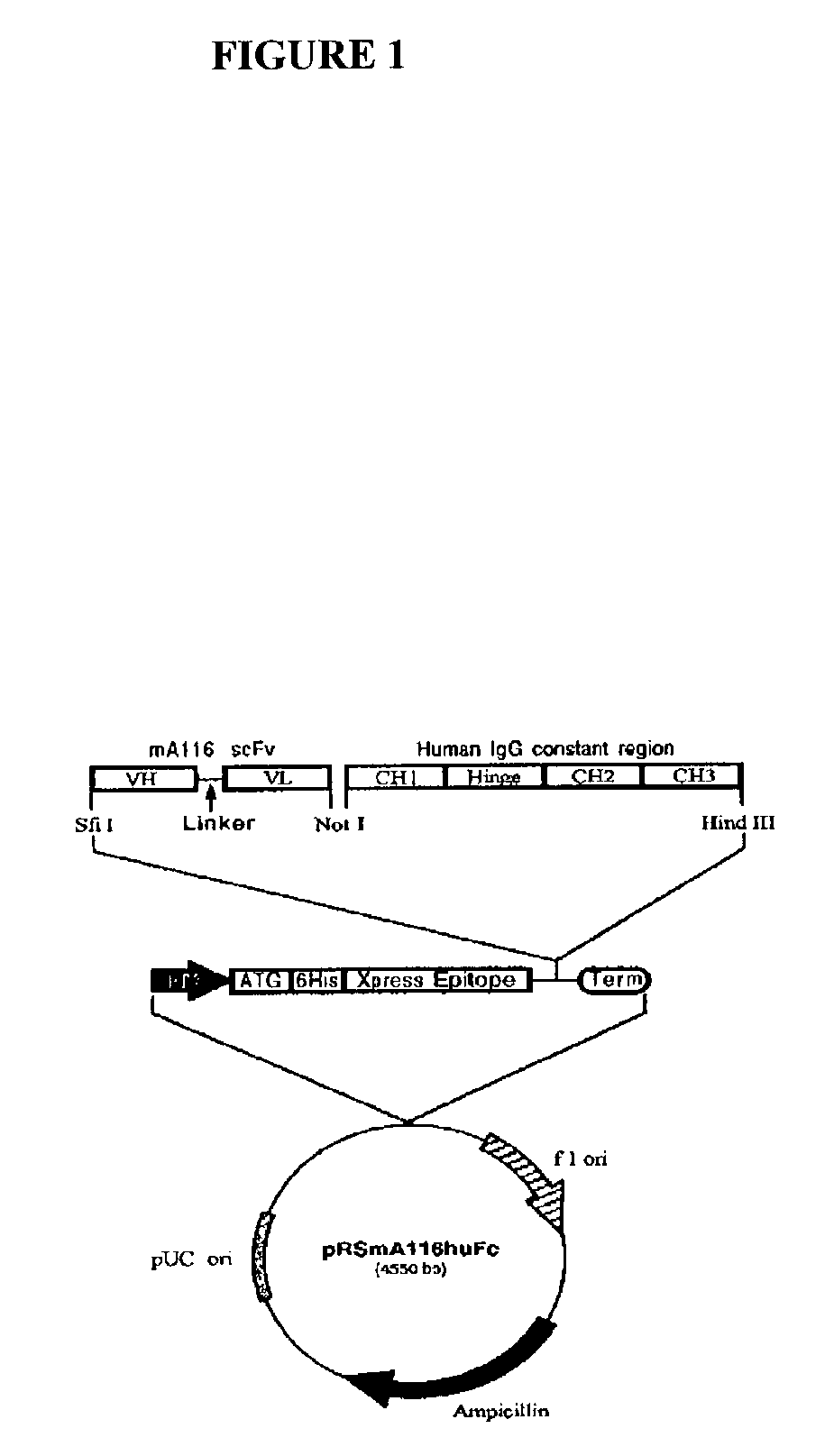
Fusion protein of human IgG1 heavy chain constant region and scFv antibody against equine encephalitis virus
InactiveUS7622111B2Low immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsInclusion bodiesScFv Antibodies
Construction of a recombinant gene fusion encoding a human IgG1 heavy chain constant region and a single-chain variable fragment antibody of 1A4A1 monoclonal antibody is disclosed. The recombinant antibody of the present invention confers human immune effector functions on murine antibodies. After expression in bacteria as inclusion bodies, the recombinant antibody was purified and refolded in vitro. The recombinant soluble antibody retains high antigen-binding affinity to VEE and possesses some human IgG crystallizable fragment domain functions. On non-reducing gel electrophoresis analysis, disulfide bond formation was found in the hinge region of the recombinant antibody. The present invention shows that the recombinant antibody is in a native, functionally active form and it provides the basis to characterize the recombinant antibody for efficacy in vivo.
Owner:THE MIN OF NAT DEFENCE
Antiviral Compounds
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and respiratory viruses including the common cold.
Owner:PROSETTA ANTIVIRAL
Antiviral Compounds
ActiveUS20120270854A1Easy to PlatingEliminate residual PBS.BiocideOrganic chemistryViral infectionWest Nile virus RNA
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and Hepatitis C.
Owner:PROSETTA ANTIVIRAL
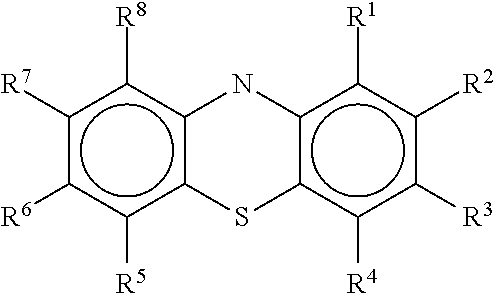
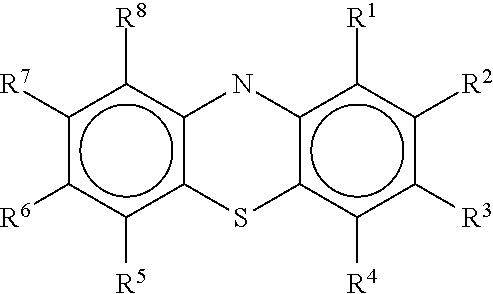
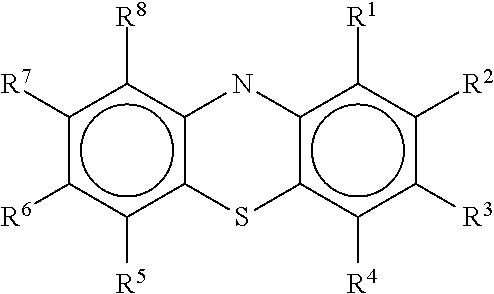
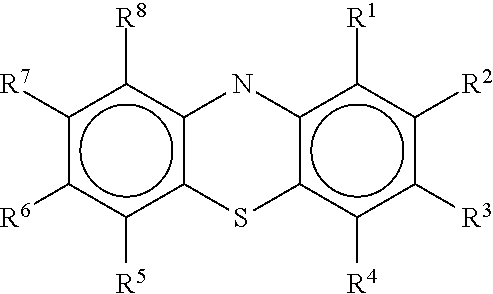
Diamino-phenothiazinyl derivatives as antiviral treatments
Owner:PROSETTA CORP
Equine Encephalitis Virus Vaccines and Methods of Using Thereof
ActiveUS20150056230A1% survivabilitySsRNA viruses positive-senseViral antigen ingredientsEquine EncephalitisVenezuelan equine encephalitis virus
Disclosed herein are nucleotide sequences which encode a plurality of structural proteins, except the capsid, of an equine encephalitis virus, wherein the nucleotide sequence is codon-optimized for mammalian expression. The nucleotide sequences are codon-optimized for expression in humans. As disclosed herein, the nucleotide sequences confer protection against Venezuelan equine encephalitis virus (VEEV), western equine encephalitis virus (WEEV), and / or eastern equine encephalitis virus (EEEV).
Owner:THE UNITED STATES OF AMERICA AS REPESENTED BY THE SEC OF ARMY ON BEHALF OF THE U S ARMY MEDICAL RES INST OF INFECTIONS DISEASES
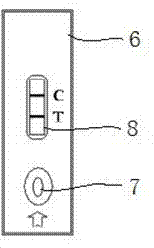
Gold mark immune paper test strip used for detecting equine encephalitis viral antibody and preparation method and application thereof
InactiveCN102445536AEasy to operateShort detection timeMaterial analysisViral antibodyEquine Encephalitis
The invention discloses a gold mark immune paper test strip used for detecting equine encephalitis viral antibody and a preparation method and an application thereof. The gold mark immune paper test strip can detect eastern equine encephalitis virus, western equine encephalitis virus and venezuelan equine encephalitis virus antibodies at the same time. The invention also discloses a preparation method and application of a gold mark immune chromatographic system which can realize respective quantitative detection, comprising preparation of the gold mark immune test paper strip and application of quantitative detection by virtue of a gold mark analysis meter. The invention combines technique principles of gold mark and immunochromotographic technique, ensures sensitivity and specificity of a detection method and has the advantages that operation is simple, detection time is short, no professional is required, the gold mark immune paper test strip is applicable to rapid field screening and the aim of quantitative detection can be achieved.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Antiviral compounds
InactiveUS8809317B2Easy to PlatingEliminate residual PBS.BiocideOrganic chemistryMedicineViral infection
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and Hepatitis C.
Owner:PROSETTA ANTIVIRAL
Methods of treating and prophylactically protecting mammalian patients infected by viruses classified in Baltimore group V
ActiveUS9919034B2Inhibition formationBiocideSugar derivativesRabiesMiddle East respiratory syndrome coronavirus
Viral infections in mammals can be treated and prophylactically prevented by systemic administration of ranpirnase and three other ribonucleases that are highly homologous with it and that have activities that are highly similar to it. Experimental results against rabies, Middle East Respiratory Syndrome Coronavirus (“MERS-CoV”), influenza, Ebola virus, Chikungunya virus, Venezuelan equine encephalitis, canine parvovirus, adenovirus-2, respiratory syncytial virus, rhinovirus-14, and vaccinia are disclosed.
Owner:ORGENESIS INC
Monoclonal antibody EEEV-6E2 resisting EEEV E2 protein, B-cell epitope peptide recognized by EEEV-6E2 as well as application of EEEV-6E2 and B-cell epitope peptid
The invention discloses a monoclonal antibody EEEV-6E2 resisting EEEV (Eastern Equine Encephalitis Virus) E2 protein, B-cell epitope peptide recognized by EEEV-6E2 as well as the application of the EEEV-6E2 and the B-cell epitope peptide. The invention further discloses a hybridoma cell strain which can stably secrete the EEEV-6E2 resisting the EEEV E2 protein and is named as EEEV-6E2. The microbial preservation number of the hybridoma cell strain is CGMCC No. 7007. An idiosyncratic reaction can occur between the EEEV-6E2 secreted by the hybridoma cell strain and the EEEV E2 protein while the EEEV-6E2 does not react with WEEVes / VEEVes (Western Equine Encephalitis Viruses / Venezuelan Equine Encephalitis Viruses). Secondly, the invention further discloses the B-cell epitope peptide which is specially recognized by the EEEV-6E2. The EEEV-6E2 and the specific B-cell epitope peptide, recognized by the EEEV-6E2, of the EEEV E2 protein can be used for preparing reagents for identifying and diagnosing EEEVes and other alphaviruses and lay a foundation for creating identifying and diagnosing methods of EEEV antigen clusters and other alphaviruses as well as further studying prevention and treatment measures.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
N4-hydroxycytidine monohydrochloride and crystal form C thereof, and preparation method and application of N4-hydroxycytidine monohydrochloride
PendingCN113956311AImprove stabilityGood medicineSugar derivativesOrganic chemistry methodsRespiratory syncytial virus (RSV)Disease cause
The invention discloses N4-hydroxycytidine monohydrochloride, a crystal form C and a preparation method thereof. The N4-hydroxycytidine monohydrochloride has the effect of preventing and treating infection of various viruses including new coronal pneumonia and is shown in a formula I. N-hydroxycytidine and hydrochloric acid are subjected to a salt forming reaction, a crystallization solvent is added after a solvent is removed, and the N4-hydroxycytidine monohydrochloride is prepared through dissolving, crystallizing, filtering and drying. The N4-hydroxycytidine monohydrochloride and the crystal form C thereof have relatively good stability and druggability, can form a pharmaceutical composition and a preparation thereof, and exert economic and social benefits in prevention and treatment of novel coronavirus infection; and the invention also discloses a pharmaceutical composition and application of the N4-hydroxycytidine monohydrochloride and the crystal form C of the N4-hydroxycytidine monohydrochloride. The compound is used for preparing medicines for preventing or treating symptoms or diseases caused by respiratory syncytial virus, influenza virus, chikungunya fever virus, Ebola virus, Venezuela equine encephalitis virus, Eastern or western equine encephalitis virus, coronavirus or Zika virus.
Owner:SUZHOU LIXIN PHARMA
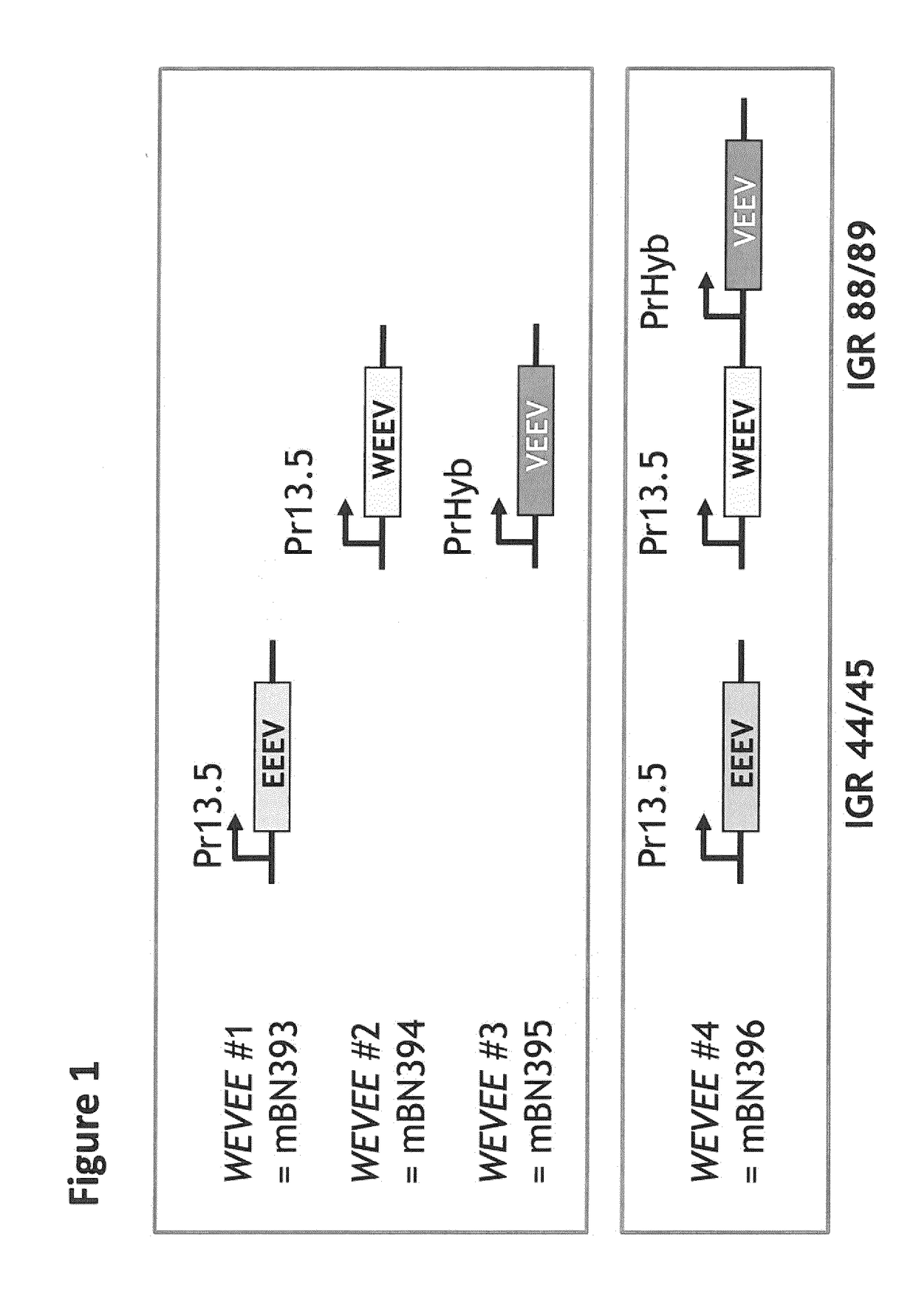
Recombinant modified vaccinia virus ankara (MVA) equine encephalitis virus vaccine
ActiveUS20190038739A1Reduce the severity of the diseaseLimiting and preventing developmentSsRNA viruses positive-senseViral antigen ingredientsDiseaseNucleotide
The present invention relates to recombinant modified vaccinia virus Ankara (MVA) and to methods of using the same. In particular, the invention relates to recombinant MVA comprising a nucleotide sequence encoding for a structural protein of an equine encephalitis virus (EEV) excluding encoding for a capsid protein of the EEV, a composition in particular a pharmaceutical composition, a vaccine or kit comprising the recombinant MVA, uses and methods thereof e.g., suitable for treating and / or preventing a western, Venezuelan, and / or eastern equine encephalitis virus caused disease.
Owner:BAVARIAN NORDIC AS
Antiviral compounds
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and respiratory viruses including the common cold.
Owner:PROSETTA ANTIVIRAL
Nucleic acid composition for detecting African horse pestivirus and kit thereof
PendingCN113061664ANo cross reactionAchieve quality controlMicrobiological testing/measurementDNA/RNA fragmentationEquine EncephalitisPathogen
The invention discloses a nucleic acid composition for detecting African horse pestivirus and a kit thereof, and belongs to the technical field of virus in-vitro nucleic acid detection. The nucleic acid composition comprises an upstream primer, a downstream primer and a detection probe, the sequence of the upstream primer is as shown in SEQ ID NO.1, the sequence of the downstream primer is as shown in SEQ ID NO.2, and the sequence of the detection probe is as shown in SEQ ID NO.3. The kit containing the nucleic acid composition can specifically detect the African horse pestivirus in a sample to be detected, and has no cross reaction with eight pathogens such as equine encephalitis virus; the kit has high sensitivity (up to 10 < 3 > copies / mL), good repeatability and controllable quality, and the whole RT-PCR detection process is easy to operate, short in detection time and high in detection efficiency.
Owner:百沃特(天津)生物技术有限公司
Recombinant modified vaccinia virus Ankara (MVA) equine encephalitis virus vaccine
The present invention relates to recombinant modified vaccinia virus Ankara (MVA) and to methods of using the same. In particular, the invention relates to recombinant MVA comprising a nucleotide sequence encoding for a structural protein of an equine encephalitis virus (EEV) excluding encoding for a capsid protein of the EEV, a composition in particular a pharmaceutical composition, a vaccine or kit comprising the recombinant MVA, uses and methods thereof e.g., suitable for treating and / or preventing a western, Venezuelan, and / or eastern equine encephalitis virus caused disease.
Owner:BAVARIAN NORDIC AS
Antiviral compounds
ActiveUS8828986B2Easy to PlatingEliminate residual PBS.BiocideOrganic chemistryVenezuelan equine encephalitis VEEViral infection
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and Hepatitis C.
Owner:PROSETTA ANTIVIRAL
Methods for detecting Venezuelan equine encephalitis virus TC-83 and its use as a biological agent simulant
The present invention is generally related to products and methods that facilitate the use of Venezuelan equine encephalitis (VEE) virus TC-83 (TC-83) as a non-hazardous simulant, or surrogate, for viable pathogenic viruses. Specifically, TC-83 nucleic sequences are used in a method of detecting VEE or TC-83 in a sample thought to contain a biological threat agent. TC-83 and its nucleic acid sequence may therefore be used in the research, development, testing, evaluation, and training for technologies that enable the detection of biological threat agents. More particularly, specific primers and probes may be used to verify that instruments and systems using PCR detection methods are functioning properly.
Owner:UNITED STATES OF AMERICA
Monoclonal antibody (eeev-6e2) against Eastern equine encephalitis virus e2 protein and its recognized B-cell epitope and application
InactiveCN103421745BViral antigen ingredientsImmunoglobulins against virusesAntigenDifferential diagnosis
The invention discloses a monoclonal antibody EEEV-6E2 resisting EEEV (Eastern Equine Encephalitis Virus) E2 protein, B-cell epitope peptide recognized by EEEV-6E2 as well as the application of the EEEV-6E2 and the B-cell epitope peptide. The invention further discloses a hybridoma cell strain which can stably secrete the EEEV-6E2 resisting the EEEV E2 protein and is named as EEEV-6E2. The microbial preservation number of the hybridoma cell strain is CGMCC No. 7007. An idiosyncratic reaction can occur between the EEEV-6E2 secreted by the hybridoma cell strain and the EEEV E2 protein while the EEEV-6E2 does not react with WEEVes / VEEVes (Western Equine Encephalitis Viruses / Venezuelan Equine Encephalitis Viruses). Secondly, the invention further discloses the B-cell epitope peptide which is specially recognized by the EEEV-6E2. The EEEV-6E2 and the specific B-cell epitope peptide, recognized by the EEEV-6E2, of the EEEV E2 protein can be used for preparing reagents for identifying and diagnosing EEEVes and other alphaviruses and lay a foundation for creating identifying and diagnosing methods of EEEV antigen clusters and other alphaviruses as well as further studying prevention and treatment measures.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
N4-hydroxycytidine monohydrate and crystal form B thereof and preparation method and application thereof
PendingCN114085259AImprove stabilityGood medicineOrganic active ingredientsSugar derivativesRespiratory syncytial virus (RSV)Disease cause
The invention discloses an N4-hydroxycytidine monohydrate and a crystal form B thereof and a preparation method thereof, the N4-hydroxycytidine monohydrate has the effect of preventing and treating infection of various viruses including novel coronavirus pneumonia and is shown in a formula I. The crystal form B of the N4-hydroxycytidine monohydrate is obtained by dissolving and crystallizing N4-hydroxycytidine in a solvent and then filtering and drying. The N4-hydroxycytidine monohydrate and the crystal form B thereof have better stability and druggability, so that a pharmaceutical composition and a preparation thereof can be formed, and economic and social benefits of the N4-hydroxycytidine monohydrate and the crystal form B thereof in prevention and treatment of novel coronavirus infection are achieved; the invention also discloses a pharmaceutical composition and application of the N4-hydroxycytidine monohydrate and the crystal form B of the N4-hydroxycytidine monohydrate, and is used for preparing medicines for preventing or treating symptoms or diseases caused by respiratory syncytial virus, influenza virus, chikungunya fever virus, Ebola virus, Venezuela equine encephalitis virus, Eastern or western equine encephalitis virus, coronavirus or Zika virus.
Owner:SUZHOU LIXIN PHARMA
Monoclonal antibody (EEEV-5F4) resisting EEEV I E2 protein, B-cell epitope peptide recognized by EEEV-5F4 as well as application of EEEVI-5F4 and B-cell epitope peptide
InactiveCN103421744AViral antigen ingredientsImmunoglobulins against virusesAntigenEastern equine encephalitis virus
The invention discloses a monoclonal antibody (EEEV-5F4) resisting an EEEV I (Eastern Equine Encephalitis Virus I) E2 protein, a B-cell epitope peptide recognized by the EEE-5F4 as well as the application of the EEEV-5F4 and the B-cell epitope peptide. The invention further discloses a hybridoma cell strain which can stably secrete the EEEV-5F4 resisting the EEEV I E2 protein and is named as EEEV-5F4. The microbial preservation number of the hybridoma cell strain is CGMCC No. 7006. An idiosyncratic reaction can occur between the EEEV-5F4 secreted by the hybridoma cell strain and the EEEV I E2 protein while the EEEV-5F4 does not react with other antigen types of EEEV antigen clusters and WEEVes / VEEVes (Western Equine Encephalitis Viruses / Venezuelan Equine Encephalitis Viruses). Secondly, the invention further discloses the B-cell epitope peptide which is specially recognized by the EEEV-5F4. The EEEV-5F4 and the specific B-cell epitope peptide, recognized by the EEEV-5F4, of the EEEV I E2 protein can be used for preparing reagents for identifying and diagnosing different antigen types of EEVA antigen clusters and lay a foundation for creating identifying and diagnosing methods of different antigen types of the EEVA antigen clusters.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Covalent inhibitors of equine encephalitis virus
Embodiments and methods for a new class of potent non-peptidic covalent inhibitors of nsP2 cysteine protease that inhibit Venezuelan equine encephalitis virus's (VEEV) replication in neuroblasts are disclosed. More particularly, an acrylate and vinyl sulfone-based chemical series were investigated as promising starting scaffolds against VEEV and as inhibitors of the cysteine protease domain of VEEV's non-structural protein 2 (nsP2). The invention discloses compounds of Formula I and analogues for treatment of VEEV.
Owner:JACKSON STATE UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com