N4-hydroxycytidine monohydrate and crystal form B thereof and preparation method and application thereof
A technology of hydroxycytidine and monohydrate, which is applied in the field of N4-hydroxycytidine hydrate and its crystal form B and preparation, and can solve the related reports on the research on polymorphism, crystal hydrate and its base that have not been found yet, etc. problem, to achieve good medicinal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
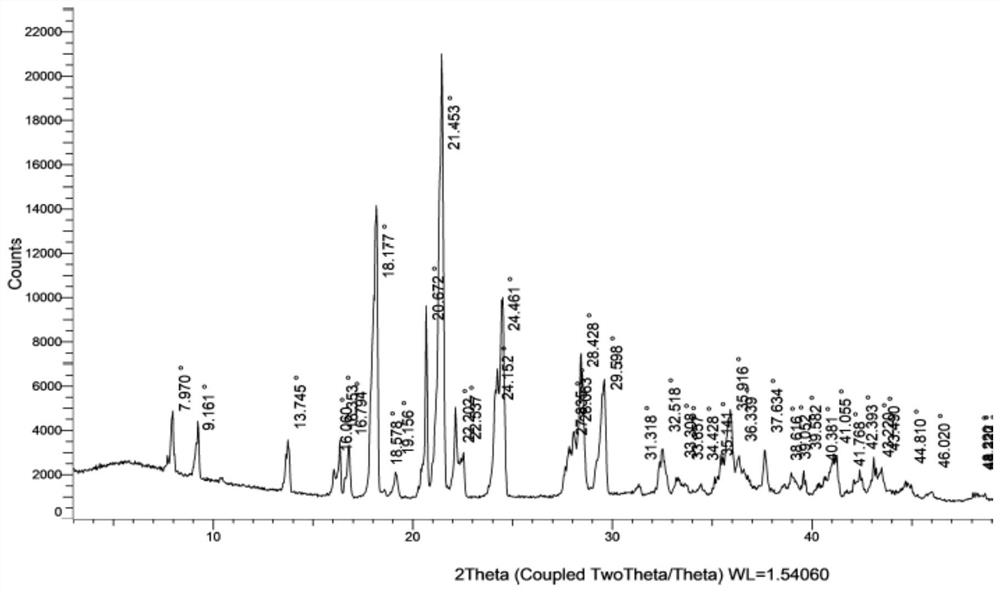
[0055] Add 5.0g of N4-hydroxycytidine and 120g of water to the reaction bottle in turn, start stirring, gradually raise the temperature to 55-65°C until completely dissolved, slowly cool down, the solution appears turbid, continue to cool down to 3-5°C, and fully crystallize for 3~ After 4 hours, filter, wash the filter cake with cold water, and dry under normal pressure at 45-55°C for 6-7 hours to obtain 4.5 g of white solid of N4-hydroxycytidine monohydrate, the molar yield is 84.2%, and the moisture content is 6.7%; X X-ray powder diffraction spectrum (XRPD) as attached figure 1 Shown, differential scanning calorimetry analysis and thermogravimetric analysis curve (DSC-TGA) as attached figure 2 shown.
Embodiment 2
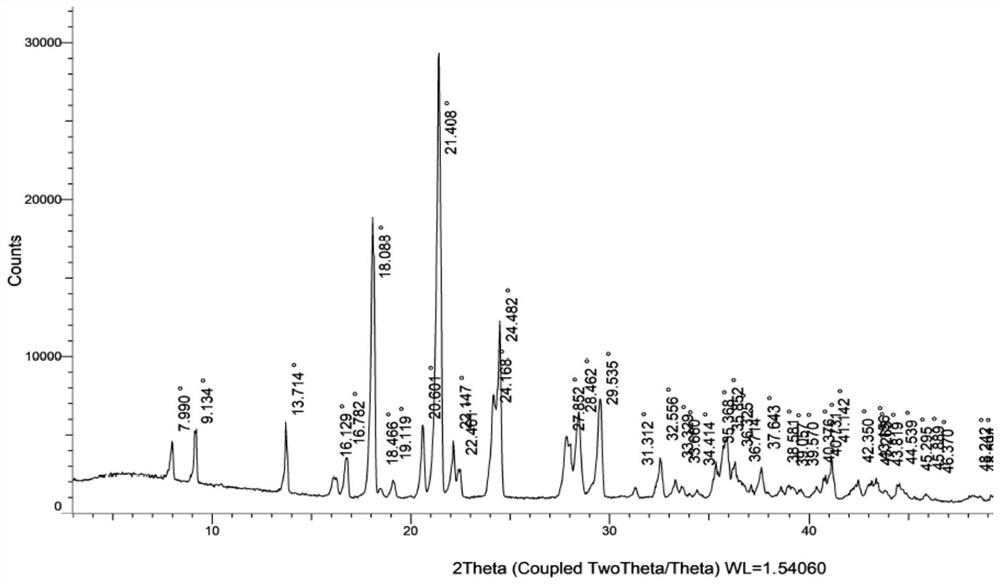
[0057] Add 5.0g of N4-hydroxycytidine, 120g of a mixed solvent of methanol and water (among them: 100g of methanol and 20g of water) into the reaction flask in sequence, start stirring, gradually raise the temperature to 45-55°C until it is completely dissolved, slowly cool down, and the solution When turbidity appears, continue to cool down to 0-5°C, fully crystallize for 3-4 hours, filter, wash the filter cake with cold methanol, and dry at 45-55°C under normal pressure for 4-5 hours to obtain the white color of N4-hydroxycytidine monohydrate Solid 4.2g, molar yield 78.5%, moisture determination 6.5%; X-ray powder diffraction spectrum (XRPD) as attached image 3 shown.
Embodiment 3
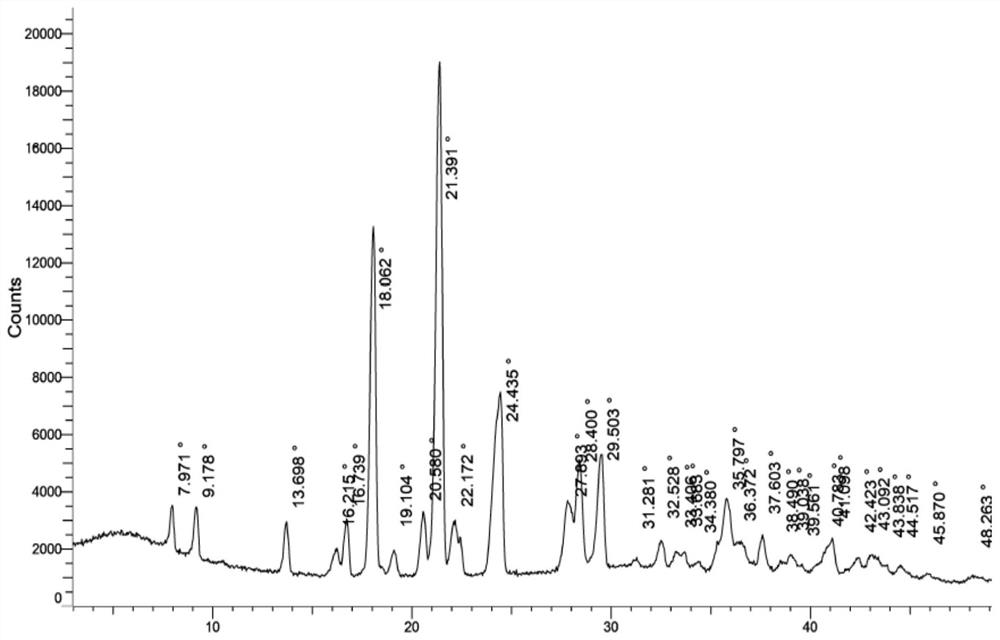
[0059] Add 5.0g of N4-hydroxycytidine, 120g of mixed solvent of ethanol and water (among them: 100g of ethanol and 20g of water) into the reaction flask in sequence, start stirring, gradually raise the temperature to 55-65°C until completely dissolved, slowly cool down, and the solution When turbidity appears, continue to cool down to 0-5°C, fully crystallize for 3-4 hours, filter, wash the filter cake with cold ethanol, and dry at 45-55°C under normal pressure for 5-6 hours to obtain the white color of N4-hydroxycytidine monohydrate Solid 4.1g, molar yield 76.7%, moisture determination 6.8%; X-ray powder diffraction spectrum (XRPD) as attached Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com