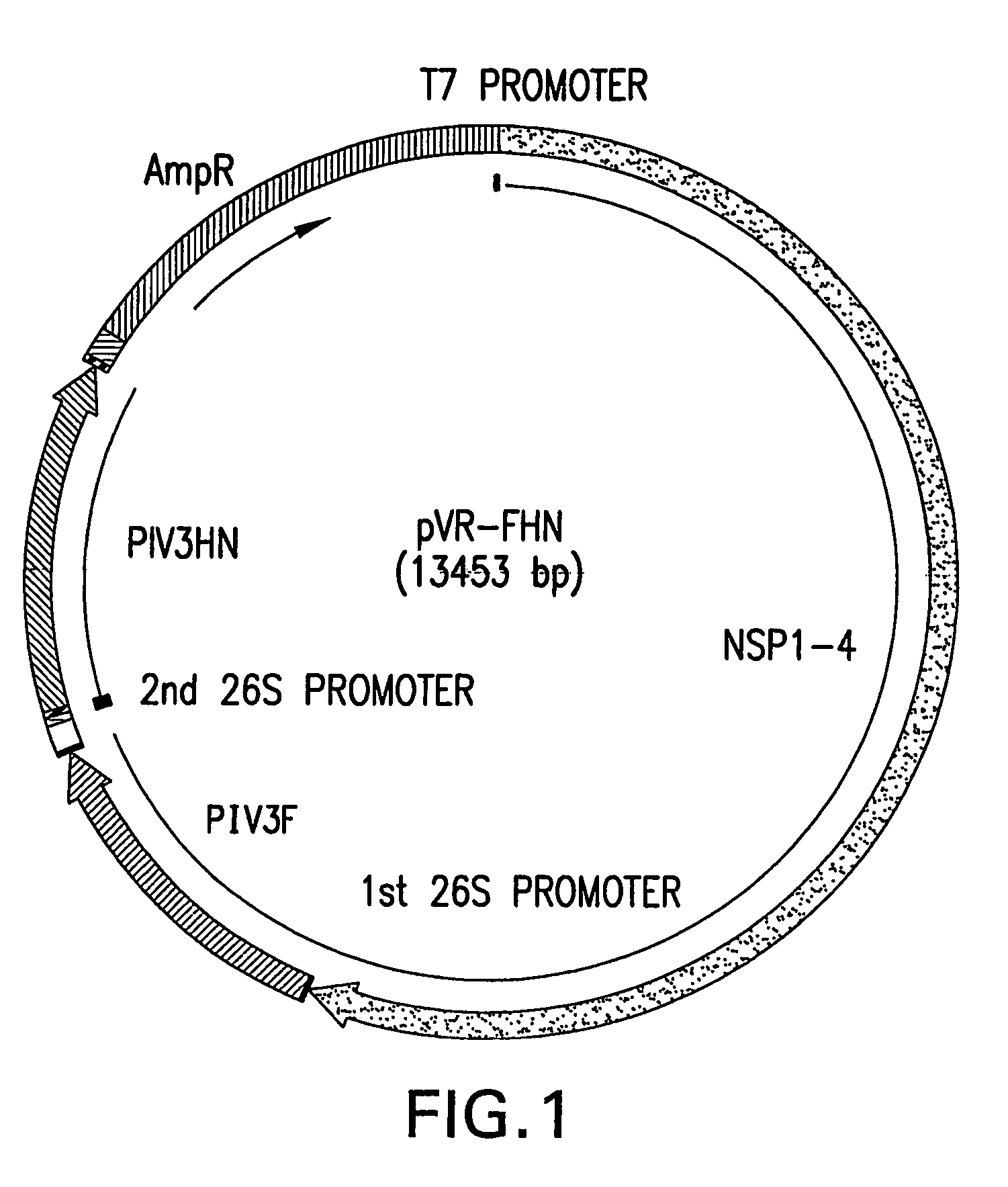
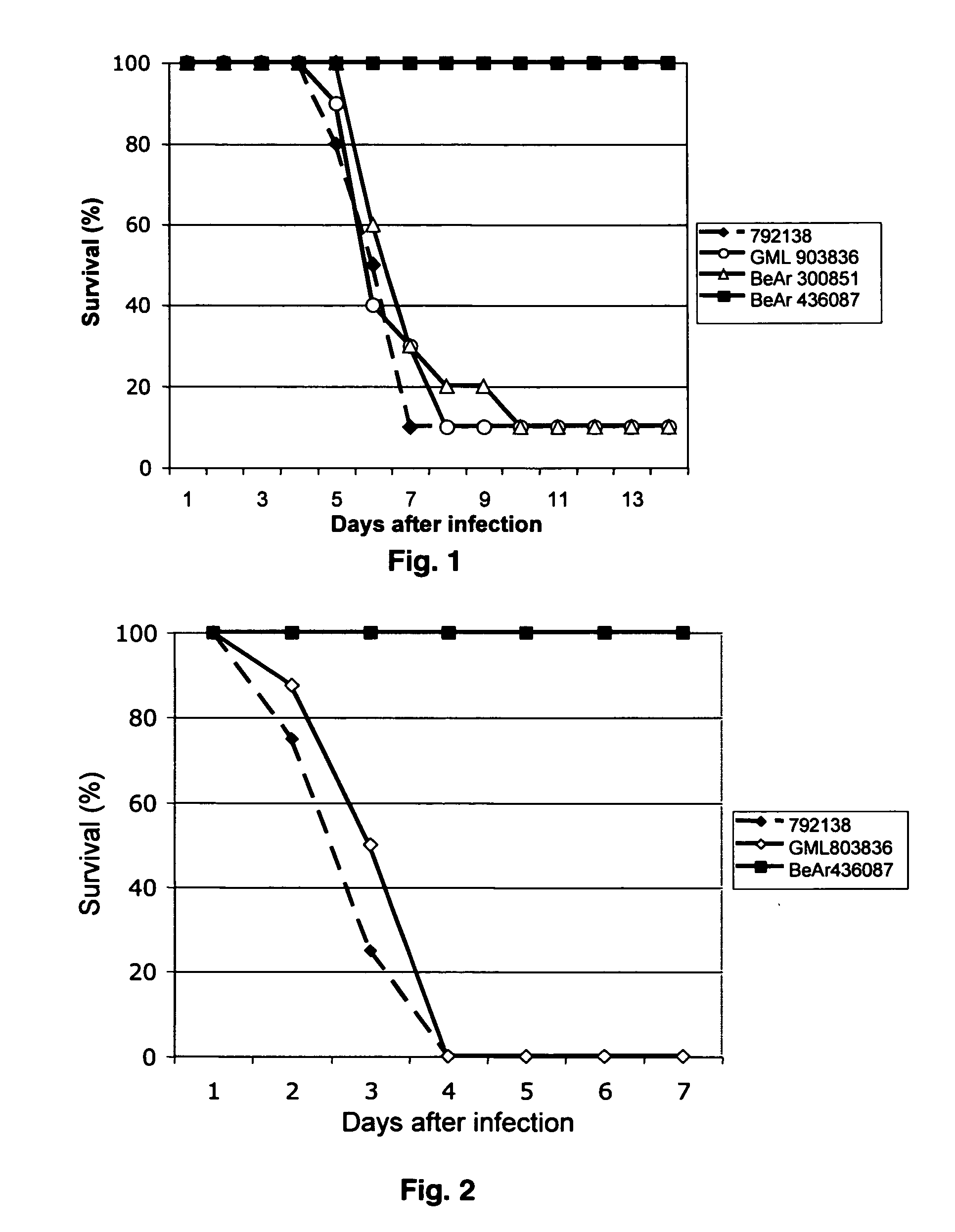
Patents
Literature
38 results about "Equine encephalosis virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Equine encephalosis virus (EEV) is a species of virus the Orbivirus genus, and a member of the Reoviridae family, related to African horse sickness virus (AHSV) and Bluetongue virus (BTV). First described in South Africa over a hundred years ago by Arnold Theiler, EEV is the causative agent of equine encephalosis (EE), an arthropod-borne disease transmitted by the Culicoides spp. midges affecting all equids. Since then the disease has become both widespread and prevalent, taking on epidemic proportions in certain parts of the country. Serological studies estimated a presence of anti-EEV antibodies in over 75% of all South African horses.
DNA-based vaccine against the encephalitis alphaviruses
This invention relates to the development of a mammalian expression vector, under which expression of the structural genes of western equine encephalitis virus have been placed under the control of an eucaryotic promoter. When the recombinant vector is administered to mammalian cell culture or using a cell-free transcription / translation system, in vitro, authentic structural proteins of western equine encephalitis virus are produced as verified by reactivity with monoclonal antibodies developed to western equine encephalitis virus. When the recombinant DNA molecule is administered in vivo, a protective immune response is induced, thereby enhancing protection of the individual against subsequent infection by western equine encephalitis virus. In a similar manner, DNA vaccines to related alphaviruses (Venezuelan and eastern equine encephalitis viruses) could also be developed.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Chimeric sindbis-western equine encephalitis virus and uses thereof
The present invention discloses a chimeric alphavirus comprising a Sindbis virus cDNA fragment, an Eastern equine encephalitis virus cDNA fragment, a Western equine encephalitis virus cDNA fragment or a combination thereof. The present also discloses the use of this chimeric alphavirus as vaccines and in serological and diagnostic assays.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
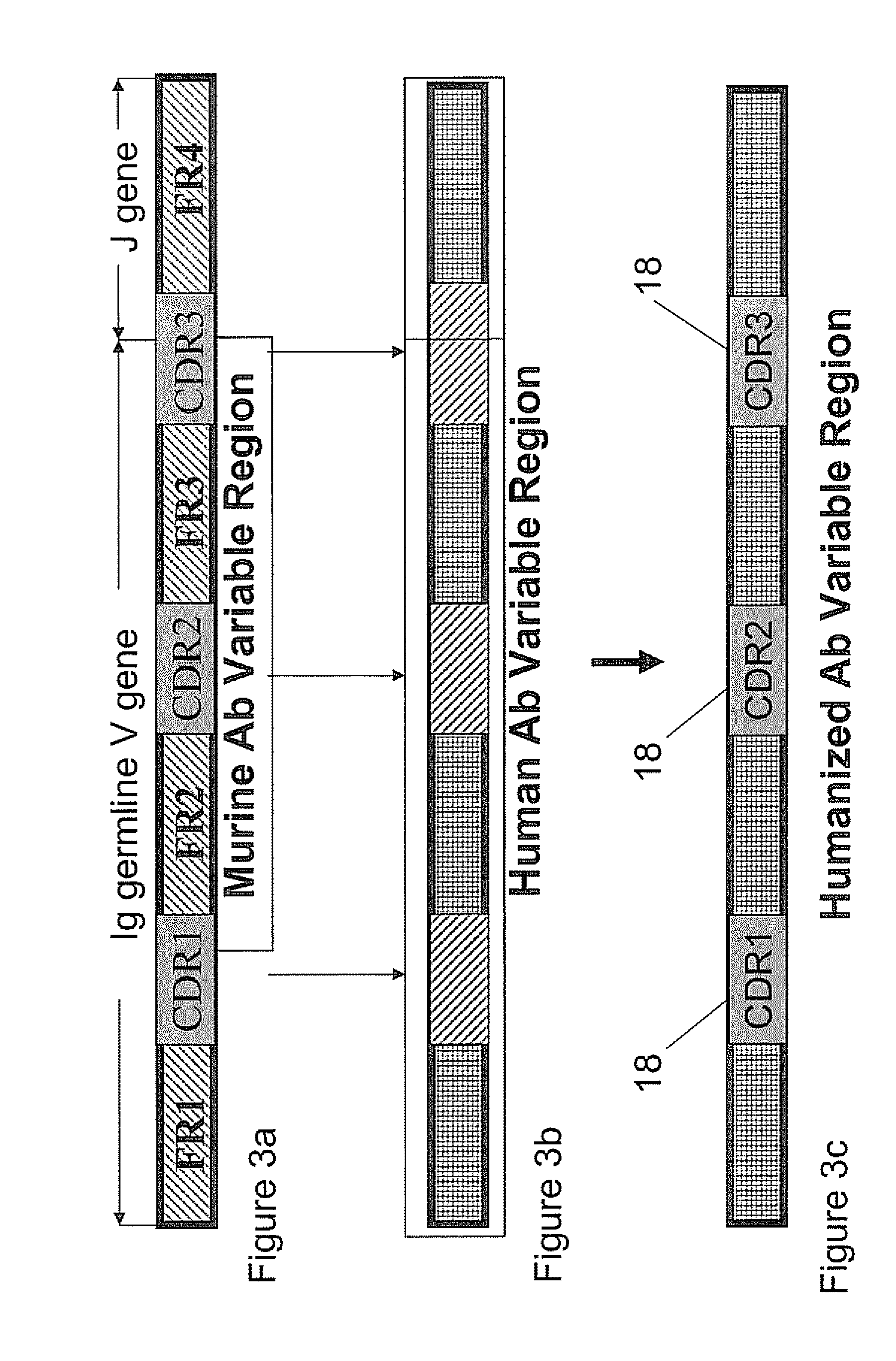
Humanized Anti-venezuelan equine encephalitis virus recombinant antibody
A CDR grafted humanized rAb comprises a human Ig framework having CDRs from murine mAb 1A4A1 VH and VL. DNA sequences and vectors incorporating such sequences are also provided as are pharmaceutical preparations and methods of using the humanized rAbs.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Nucleic acid for encoding SARS-CoV-2 virus spike protein and application of nucleic acid
ActiveCN112226445ASsRNA viruses positive-senseViral antigen ingredientsTGE VACCINEEquine encephalosis virus
The invention relates to the technical field of biological medicine, in particular to nucleic acid for encoding SARSCoV2 virus spike protein and application of the nucleic acid. Through codon optimization, the invention provides the Spike high-expression novel coronavirus mRNA vaccine based on the TC83Venezuelan Equine Encephalosis Virus (VEEV) in the alpha virus family, and the vaccine can detecta spike antibody after 14 days of inoculation in an animal experiment.
Owner:成都新诺明生物科技有限公司 +1
Venezuelan equine encephalitis virus replicons with adaptive mutations in the genome and uses thereof
ActiveUS7332322B2Reduced cytopathic effectReduce productionSsRNA viruses positive-senseViral antigen ingredientsMammalViral replication
The present invention provides a Venezuelan equine encephalitis virus replicon RNA useful in the development of stable lines of mammalian, avian and insect cells in which these replicons will persistently replicate. Venezuelan equine encephalitis (VEE) virus replicons contain a number of unique adaptive mutations that make the replicons noncytopathic. The replicons remain resistant to IFN-α / β. Replicon replication leads to high-level production of heterologous proteins, which are encoded by the replicons' genome and are under the control of a viral subgenomic promoter. Also provided are methods of screening for inhibitory compounds of Venezuelan equine encephalitis virus replication and eastern equine encephalitis virus replication.
Owner:BOARD OF RGT UNIV OF TEXAS THE
Attenuated recombinant alphaviruses incapable of replicating in mosquitoes and uses thereof
ActiveUS20110052634A1SsRNA viruses positive-senseMutant preparationEastern equine encephalitis virusEncephalitis Viruses
The present invention discloses an attenuated recombinant alphavirus that is incapable of replicating in mosquito cells and of transmission by mosquito vectors. These attenuated alphavirus may include but is not limited to Western Equine Encephalitis virus, Eastern equine encephalitis virus, Venezuelan equine encephalitis virus or Chikungunya virus. The present invention also discloses the method of generating such alphaviruses and their use as immunogenic compositions.
Novel DNA-based vaccine against the encephalitis alphaviruses
InactiveUS20050118251A1BiocideSsRNA viruses positive-senseCell freeEastern equine encephalitis virus
This invention relates to the development of a mammalian expression vector, under which expression of the structural genes of western equine encephalitis virus have been placed under the control of an eucaryotic promoter. When the recombinant vector is administered to mammalian cell culture or using a cell-free transcription / translation system, in vitro, authentic structural proteins of western equine encephalitis virus are produced as verified by reactivity with monoclonal antibodies developed to western equine encephalitis virus. When the recombinant DNA molecule is administered in vivo, a protective immune response is induced, thereby enhancing protection of the individual against subsequent infection by western equine encephalitis virus. In a similar manner, DNA vaccines to related alphaviruses (Venezuelan and eastern equine encephalitis viruses) could also be developed.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Fusogenic, self-propagating blebs as immunogenic compositions
InactiveUS7541038B2SsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
Attenuated recombinant alphaviruses incapable of replicating in mosquitoes and uses thereof
ActiveUS8426188B2Animal cellsSsRNA viruses positive-senseEastern equine encephalitis virusChikungunya
The present invention discloses an attenuated recombinant alphavirus that is incapable of replicating in mosquito cells and of transmission by mosquito vectors. These attenuated alphavirus may include but is not limited to Western Equine Encephalitis virus, Eastern equine encephalitis virus, Venezuelan equine encephalitis virus or Chikungunya virus. The present invention also discloses the method of generating such alphaviruses and their use as immunogenic compositions.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Chimeric chikungunya virus and uses thereof
ActiveUS20110171249A1Avoid infectionSsRNA viruses positive-senseSugar derivativesSindbis virusChikungunya fever
The present invention discloses a chimeric Chikungunya virus comprising a heterologous alphavirus cDNA fragment and a Chikungunya virus cDNA fragment. The heterologous alphavirus may include but is not limited to Sindbis virus, Eastern equine encephalitis virus or Venezuelan equine encephalitis virus. The present invention also discloses the use of this chimeric Chikungunya virus as vaccines and in serological and diagnostic assays.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Humanized antibodies against the venezuelan equine encephalitis virus
InactiveUS20060024666A1Prevent and neutralize viral infectionFungiBacteriaHumanized antibodyViral infection
Owner:ALEXION PHARMA INC
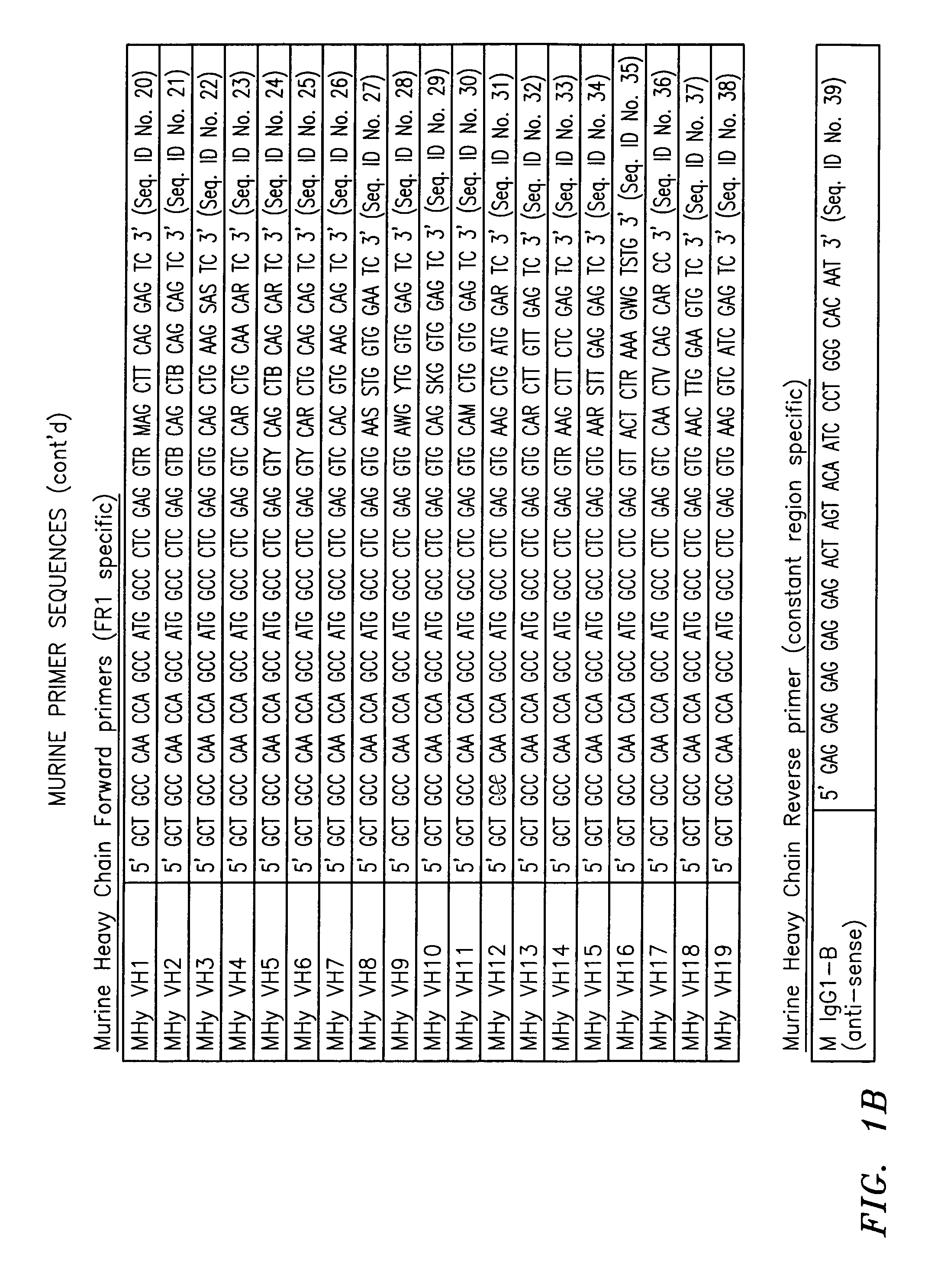
Kit for detecting eastern equine encephalitis virus and west equine encephalitis virus by real-time fluorescence quantitative RT-PCR
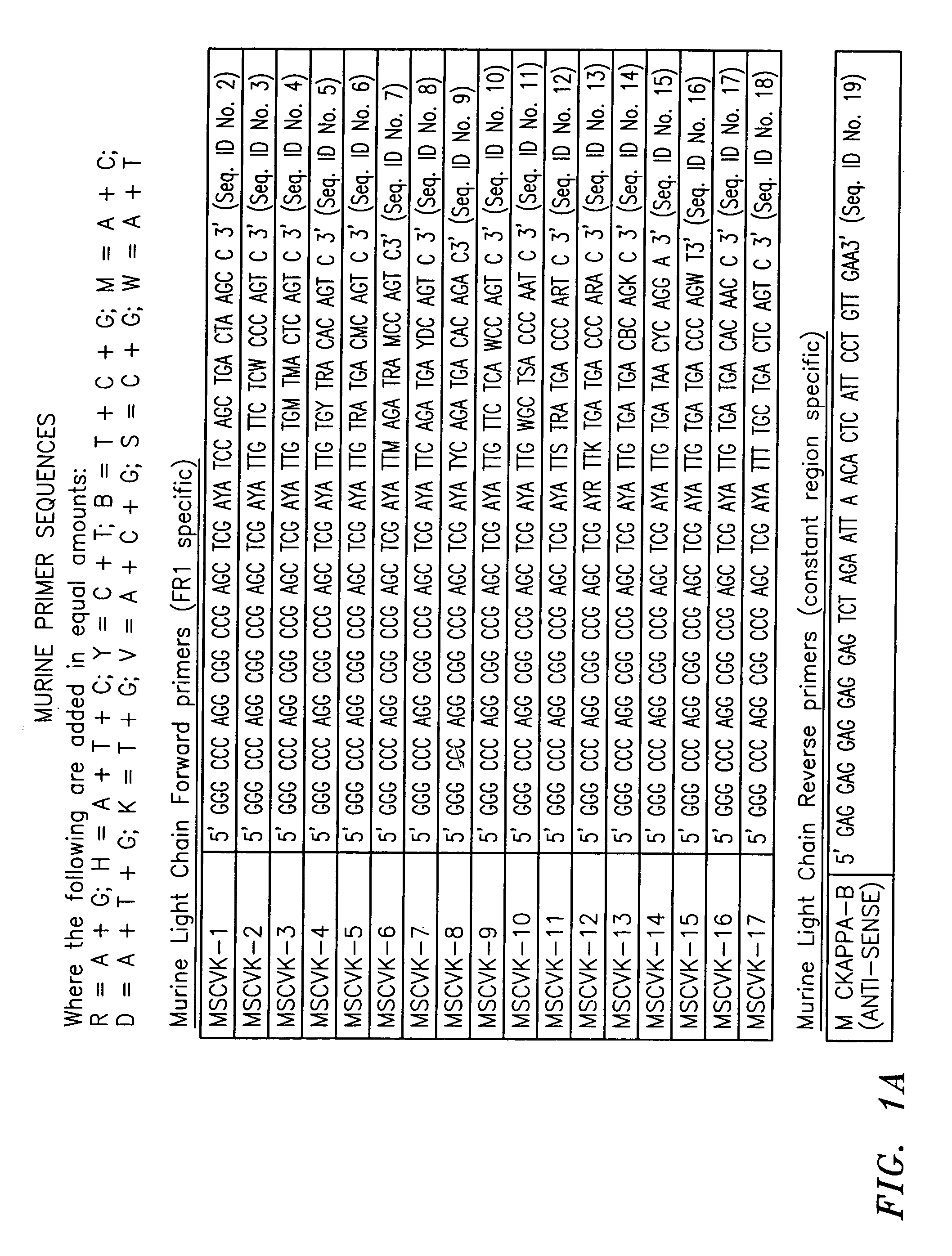
InactiveCN101967524AMicrobiological testing/measurementFluorescence/phosphorescenceForward primerFluorescence
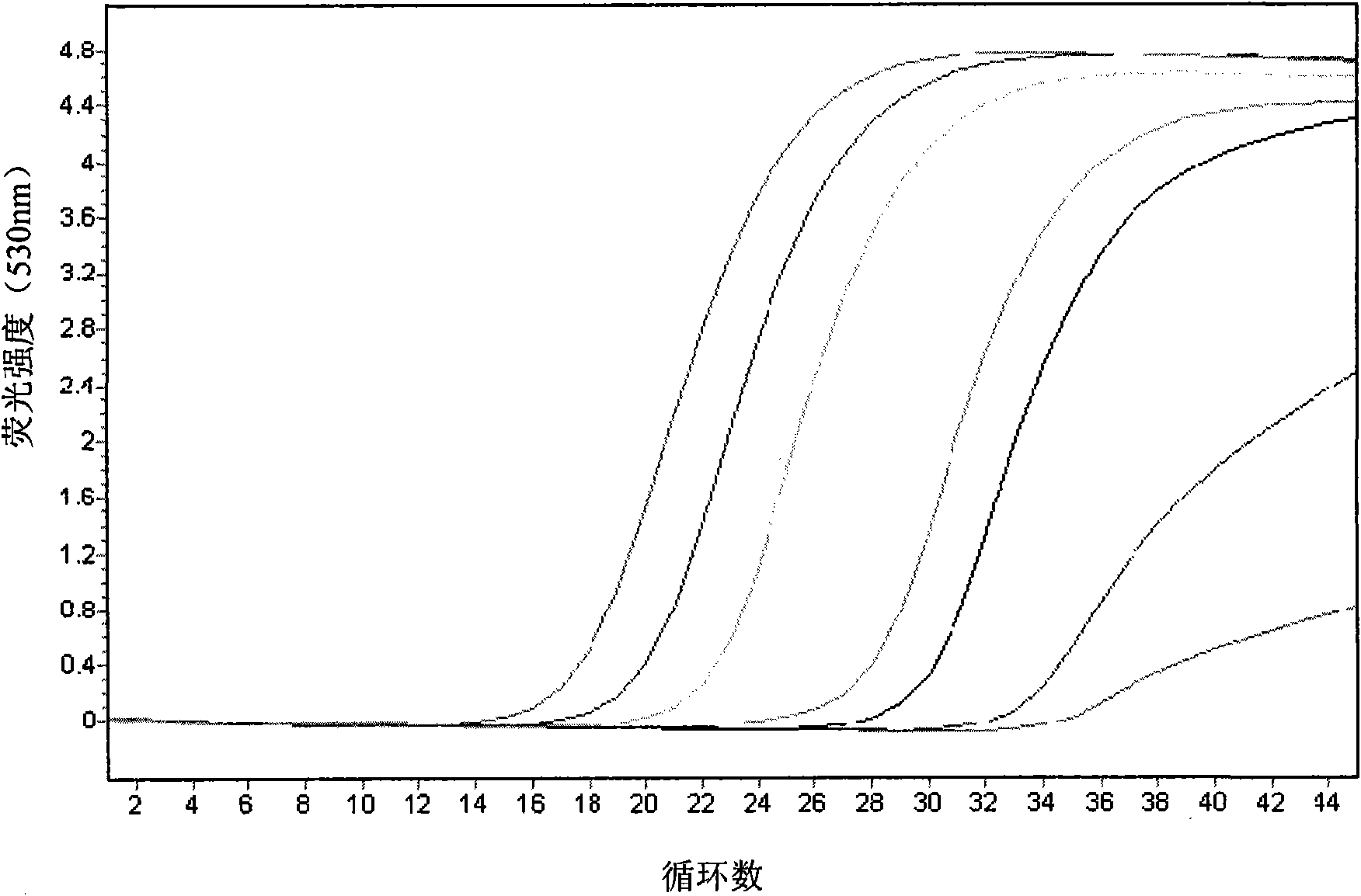
The invention discloses a kit for detecting eastern equine encephalitis virus and west equine encephalitis virus by real-time fluorescence quantitative RT-PCR. The kit provided by the invention comprises a primer pair as shown in the following (1) and (2): (1) EEEV forward primer: TGTGCGTACCTCCTCATCGTT, EEEV reverse primer: GACTGGCGTGAATCTCTGCT; (2) WEEV forward primer: AGGGATACCCCCGAAGGTT, WEEV reverse primer: GTGAATAGCACACGGGTGGTT. The kit can be applied to detecting EEEV and WEEV and has good sensitivity and specificity; in addition, the lowest EEEV virus titer that the kit can detect is 0.2TCID50 / ml and the WEEV virus titer that the kit can detect is 1TCID50 / ml.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Method and kit for detecting multiple encephalitis related viruses
ActiveCN102796827AImprove throughputAddresses issues with reduced sensitivityMicrobiological testing/measurementDNA/RNA fragmentationEastern equine encephalitis virusMicrosphere
The invention provides a method and a kit for detecting multiple encephalitis related viruses. In particular, the invention discloses a method for simultaneously detecting multiple encephalitis related viruses. The method comprises the following steps of: performing polymerase chain reaction (PCR) by using a specific primer set aiming at the encephalitis related viruses in a polymerase reaction system to obtain an amplification product; and detecting with specific probes or probe microspheres. The invention also provides the corresponding kit. The method and the kit can be used for sensitively and simply detecting and identifying multiple encephalitis related viruses comprising eastern equine encephalitis viruses, western equine encephalitis viruses, Venezuelan equine encephalitis viruses, forest encephalitis viruses and Japanese encephalitis viruses.
Owner:SHANGHAI TELLGEN LIFE SCI CO LTD
Immunogenic compositions comprising venezuelan equine encephalitis virus replicon vectors and paramyxovirus protein antigens
InactiveCN1798760ASsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
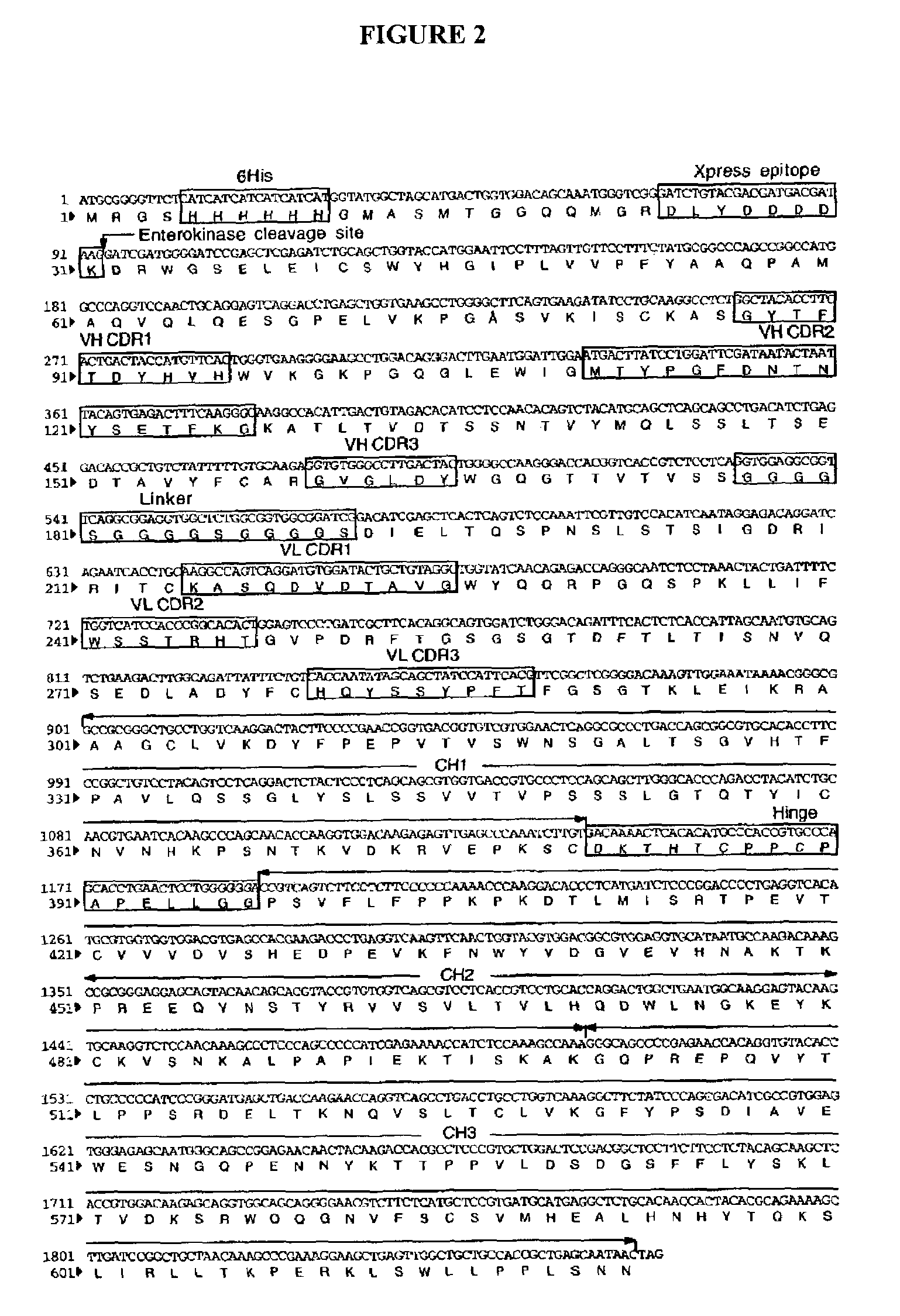
Fusion protein of human IgG1 heavy chain constant region and scFv antibody against equine encephalitis virus
InactiveUS7622111B2Low immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsInclusion bodiesScFv Antibodies
Construction of a recombinant gene fusion encoding a human IgG1 heavy chain constant region and a single-chain variable fragment antibody of 1A4A1 monoclonal antibody is disclosed. The recombinant antibody of the present invention confers human immune effector functions on murine antibodies. After expression in bacteria as inclusion bodies, the recombinant antibody was purified and refolded in vitro. The recombinant soluble antibody retains high antigen-binding affinity to VEE and possesses some human IgG crystallizable fragment domain functions. On non-reducing gel electrophoresis analysis, disulfide bond formation was found in the hinge region of the recombinant antibody. The present invention shows that the recombinant antibody is in a native, functionally active form and it provides the basis to characterize the recombinant antibody for efficacy in vivo.
Owner:THE MIN OF NAT DEFENCE
Chimeric sindbis-western equine encephalitis virus and uses thereof
ActiveUS8748591B2Avoid infectionBiocideSsRNA viruses positive-senseAssayEastern equine encephalitis virus
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Chimeric sindbis-eastern equine encephalitis virus and uses thereof
InactiveUS20100247565A1Avoid infectionSsRNA viruses positive-senseViral antigen ingredientsSindbis virusSerology
The present invention discloses a chimeric alphavirus comprising a Sindbis virus cDNA fragment and an Eastern equine encephalitis virus cDNA fragment. The present also discloses the use of this chimeric alphavirus as vaccines and in serological and diagnostic assays.
Owner:BOARD OF RGT UNIV OF TEXAS SYST THE
Encephalitis-related virus detection kit and application thereof
InactiveCN105603128AReduced risk of cross-contaminationLow costMicrobiological testing/measurementDNA/RNA fragmentationJapanese encephalitisEastern equine encephalitis virus
The invention discloses an encephalitis-related virus detection kit and application thereof. The kit is used on the basis of the principles of multiplex PCR (polymerase chain reaction) combined nucleic acid intrusive reaction and nano gold color development. The kit comprises a combination of five types of primers and probes, and can be used for simultaneously detecting eastern equine encephalitis virus, western equine encephalitis virus, epidemic encephalitis b virus, West Nile virus and Nipah virus. When being used for detecting the five viruses, the kit has the advantages of high specificity and high sensitivity, and does not need any expensive apparatus; the detection result can be observed by naked eyes; and thus, the kit can be applied to the basic level.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Genetically biotinylated recombinant antibody in immunofiltration assay by light addressable potentiometric sensor for identification of Venezuelan equine encephalitis virus
InactiveUS7309566B2Microbiological testing/measurementImmunoglobulins against virusesBiotin-streptavidin complexFiltration
A genetically biotinylated single chain fragment variable (scFv) antibody against Venezuelan equine encephalitis virus (VEE) being applied in a system consisting of an immunofiltration-enzyme assay (IFA) with a light addressable potentiometric sensor (LAPS) for the rapid identification of VEE is disclosed. The IFA entails formation of an immunocomplex sandwich consisting of VEE, biotinylated antibody, fluoresceinated antibody and streptavidin, capturing the sandwich by filtration on biotinylated membrane, and detecting the sandwich by anti-fluorescein urease conjugate. The concentration ratio of biotinylated to fluoresceinated antibodies is investigated and optimized. The IFA / LAPS assay sensitivity was approximately equal to that of a conventional enzyme-linked immunosorbant assay utilizing polystyrene plates and a chromogenic substrate, however, less time and effort were required for performance of the IFA / LAPS assay.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
N4-hydroxycytidine monohydrochloride and crystal form C thereof, and preparation method and application of N4-hydroxycytidine monohydrochloride
PendingCN113956311AImprove stabilityGood medicineSugar derivativesOrganic chemistry methodsRespiratory syncytial virus (RSV)Disease cause
The invention discloses N4-hydroxycytidine monohydrochloride, a crystal form C and a preparation method thereof. The N4-hydroxycytidine monohydrochloride has the effect of preventing and treating infection of various viruses including new coronal pneumonia and is shown in a formula I. N-hydroxycytidine and hydrochloric acid are subjected to a salt forming reaction, a crystallization solvent is added after a solvent is removed, and the N4-hydroxycytidine monohydrochloride is prepared through dissolving, crystallizing, filtering and drying. The N4-hydroxycytidine monohydrochloride and the crystal form C thereof have relatively good stability and druggability, can form a pharmaceutical composition and a preparation thereof, and exert economic and social benefits in prevention and treatment of novel coronavirus infection; and the invention also discloses a pharmaceutical composition and application of the N4-hydroxycytidine monohydrochloride and the crystal form C of the N4-hydroxycytidine monohydrochloride. The compound is used for preparing medicines for preventing or treating symptoms or diseases caused by respiratory syncytial virus, influenza virus, chikungunya fever virus, Ebola virus, Venezuela equine encephalitis virus, Eastern or western equine encephalitis virus, coronavirus or Zika virus.
Owner:SUZHOU LIXIN PHARMA
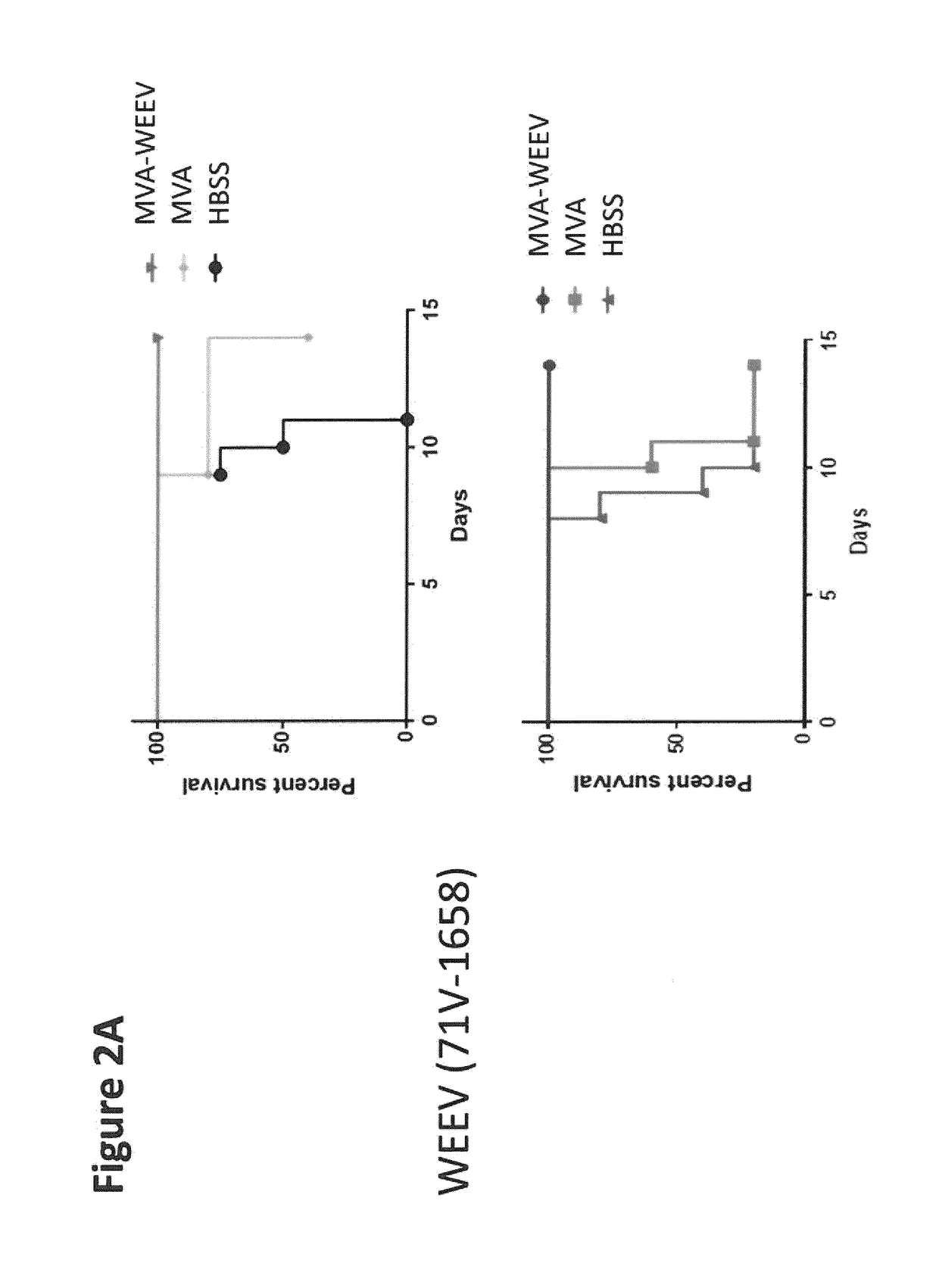
Recombinant modified vaccinia virus ankara (MVA) equine encephalitis virus vaccine
ActiveUS20190038739A1Reduce the severity of the diseaseLimiting and preventing developmentSsRNA viruses positive-senseViral antigen ingredientsDiseaseNucleotide
The present invention relates to recombinant modified vaccinia virus Ankara (MVA) and to methods of using the same. In particular, the invention relates to recombinant MVA comprising a nucleotide sequence encoding for a structural protein of an equine encephalitis virus (EEV) excluding encoding for a capsid protein of the EEV, a composition in particular a pharmaceutical composition, a vaccine or kit comprising the recombinant MVA, uses and methods thereof e.g., suitable for treating and / or preventing a western, Venezuelan, and / or eastern equine encephalitis virus caused disease.
Owner:BAVARIAN NORDIC AS
Recombinant modified vaccinia virus Ankara (MVA) equine encephalitis virus vaccine
The present invention relates to recombinant modified vaccinia virus Ankara (MVA) and to methods of using the same. In particular, the invention relates to recombinant MVA comprising a nucleotide sequence encoding for a structural protein of an equine encephalitis virus (EEV) excluding encoding for a capsid protein of the EEV, a composition in particular a pharmaceutical composition, a vaccine or kit comprising the recombinant MVA, uses and methods thereof e.g., suitable for treating and / or preventing a western, Venezuelan, and / or eastern equine encephalitis virus caused disease.
Owner:BAVARIAN NORDIC AS
Humanized anti-Venezuelan equine encephalitis virus recombinant antibodies
A CDR grafted humanized recombinant antibody against infection from Venezuelan equine encephalitis virus (VEEV) comprises a human Ig framework having CDRs from murine mAb 1A4A1 VH and VL. DNA sequences, expression vectors incorporating such sequences and transformed host cells are also provided. Also provided are pharmaceutical compositions and methods of prophylaxis and treatment against VEEV infection using the humanized recombinant antibodies of the invention.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
N4-hydroxycytidine monohydrate and crystal form B thereof and preparation method and application thereof
PendingCN114085259AImprove stabilityGood medicineOrganic active ingredientsSugar derivativesRespiratory syncytial virus (RSV)Disease cause
The invention discloses an N4-hydroxycytidine monohydrate and a crystal form B thereof and a preparation method thereof, the N4-hydroxycytidine monohydrate has the effect of preventing and treating infection of various viruses including novel coronavirus pneumonia and is shown in a formula I. The crystal form B of the N4-hydroxycytidine monohydrate is obtained by dissolving and crystallizing N4-hydroxycytidine in a solvent and then filtering and drying. The N4-hydroxycytidine monohydrate and the crystal form B thereof have better stability and druggability, so that a pharmaceutical composition and a preparation thereof can be formed, and economic and social benefits of the N4-hydroxycytidine monohydrate and the crystal form B thereof in prevention and treatment of novel coronavirus infection are achieved; the invention also discloses a pharmaceutical composition and application of the N4-hydroxycytidine monohydrate and the crystal form B of the N4-hydroxycytidine monohydrate, and is used for preparing medicines for preventing or treating symptoms or diseases caused by respiratory syncytial virus, influenza virus, chikungunya fever virus, Ebola virus, Venezuela equine encephalitis virus, Eastern or western equine encephalitis virus, coronavirus or Zika virus.
Owner:SUZHOU LIXIN PHARMA
Monoclonal antibody (EEEV-5F4) resisting EEEV I E2 protein, B-cell epitope peptide recognized by EEEV-5F4 as well as application of EEEVI-5F4 and B-cell epitope peptide
InactiveCN103421744AViral antigen ingredientsImmunoglobulins against virusesAntigenEastern equine encephalitis virus
The invention discloses a monoclonal antibody (EEEV-5F4) resisting an EEEV I (Eastern Equine Encephalitis Virus I) E2 protein, a B-cell epitope peptide recognized by the EEE-5F4 as well as the application of the EEEV-5F4 and the B-cell epitope peptide. The invention further discloses a hybridoma cell strain which can stably secrete the EEEV-5F4 resisting the EEEV I E2 protein and is named as EEEV-5F4. The microbial preservation number of the hybridoma cell strain is CGMCC No. 7006. An idiosyncratic reaction can occur between the EEEV-5F4 secreted by the hybridoma cell strain and the EEEV I E2 protein while the EEEV-5F4 does not react with other antigen types of EEEV antigen clusters and WEEVes / VEEVes (Western Equine Encephalitis Viruses / Venezuelan Equine Encephalitis Viruses). Secondly, the invention further discloses the B-cell epitope peptide which is specially recognized by the EEEV-5F4. The EEEV-5F4 and the specific B-cell epitope peptide, recognized by the EEEV-5F4, of the EEEV I E2 protein can be used for preparing reagents for identifying and diagnosing different antigen types of EEVA antigen clusters and lay a foundation for creating identifying and diagnosing methods of different antigen types of the EEVA antigen clusters.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Multiplex PCR primer and probe combination for detecting 18 pathogens and application of multiplex PCR primer and probe combination
ActiveCN114317837AConvenient and quick screeningEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationEnterovirusEastern equine encephalitis virus
The invention discloses a multiplex PCR primer and probe combination for detecting 18 pathogens and application of the multiplex PCR primer and probe combination. According to the invention, influenza A virus, influenza B virus, respiratory syncytial virus, rhinovirus, and human parainfluenza virus (differentiating type I, type II, type III, type IV, type IV, type IV, type IV, type IV, type IV, type V. A multiplex PCR (Polymerase Chain Reaction) primer and probe combination which can be used for simultaneously detecting the 18 pathogens is researched and designed by taking the 18 pathogens (type III), enterovirus, dengue virus, eastern equine encephalitis virus, western equine encephalitis virus, Venezuela equine encephalitis virus, forest encephalitis virus, Saint Lewis encephalitis virus, west Nile virus, yellow fever virus, Nipah virus and Hendea virus as targets. On the basis, a corresponding gene chip and a detection kit are developed. The primer and probe scheme designed and obtained by the invention is strong in specificity, wide in detection range and good in result accuracy, not only contributes to prevention and control of infectious pathogens, but also can be used for external quality assessment and the like of corresponding pathogens.
Owner:潮州凯普生物化学有限公司 +2
Oncolytic virus based on equine encephalitis virus and application thereof
PendingCN113717951AHigh genetic stabilitySignificant oncolytic effectSsRNA viruses positive-senseVirus peptidesCapsidTumor cells
The invention relates to an oncolytic virus based on an equine encephalitis virus and application thereof. The oncolytic virus is a Venezuela equine encephalitis virus with a function-inactivated capsid protein gene. The oncolytic virus can also be used as an expression vector for expression of exogenous genes. The oncolytic virus has a remarkable oncolytic effect in various tumour cells and mouse tumour-bearing models; an effective anti-cancer therapeutic agent can be provided for clinical tumour treatment; and the oncolytic virus has a good application prospect.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Equine encephalitis virus vaccines and methods of using thereof
ActiveUS9555090B2% survivabilitySsRNA viruses positive-senseViral antigen ingredientsNucleotideEastern equine encephalitis virus
Disclosed herein are nucleotide sequences which encode a plurality of structural proteins, except the capsid, of an equine encephalitis virus, wherein the nucleotide sequence is codon-optimized for mammalian expression. The nucleotide sequences are codon-optimized for expression in humans. As disclosed herein, the nucleotide sequences confer protection against Venezuelan equine encephalitis virus (VEEV), western equine encephalitis virus (WEEV), and / or eastern equine encephalitis virus (EEEV).
Owner:THE UNITED STATES OF AMERICA AS REPESENTED BY THE SEC OF ARMY ON BEHALF OF THE U S ARMY MEDICAL RES INST OF INFECTIONS DISEASES
A method and kit for detecting multiple encephalitis-associated viruses
ActiveCN102796827BImprove throughputAddresses issues with reduced sensitivityMicrobiological testing/measurementDNA/RNA fragmentationEastern equine encephalitis virusMicrosphere
The invention provides a method and a kit for detecting multiple encephalitis related viruses. In particular, the invention discloses a method for simultaneously detecting multiple encephalitis related viruses. The method comprises the following steps of: performing polymerase chain reaction (PCR) by using a specific primer set aiming at the encephalitis related viruses in a polymerase reaction system to obtain an amplification product; and detecting with specific probes or probe microspheres. The invention also provides the corresponding kit. The method and the kit can be used for sensitively and simply detecting and identifying multiple encephalitis related viruses comprising eastern equine encephalitis viruses, western equine encephalitis viruses, Venezuelan equine encephalitis viruses, forest encephalitis viruses and Japanese encephalitis viruses.
Owner:SHANGHAI TELLGEN LIFE SCI CO LTD
A multiplex PCR primer and probe combination for detecting pathogens and its application
ActiveCN114317837BConvenient and quick screeningEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationEnterovirusMultiplex
Owner:潮州凯普生物化学有限公司 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com