Oncolytic virus based on equine encephalitis virus and application thereof
An oncolytic virus, equine encephalitis technology, applied in the direction of viruses, viral peptides, viruses/phages, etc., to achieve good application prospects, high genetic stability, and significant oncolytic effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Example 1: Construction of the Venezuelan Equine Encephalitis Virus (VEEV) Vaccine Strain TC-83 Infectious Clones Deleting the Capsid Protein Gene and Rescue of the Virus
[0139] In this example, an infectious clone was constructed by exploring the Venezuelan Equine Encephalitis Virus (VEEV) vaccine strain TC-83 lacking the capsid protein gene.
[0140] 1. Construction of Infectious Clones Deleting All Genes of Capsid Protein (VEEV-delC)
[0141] According to the VEEV-TC83 sequence (GenBank accession no. DQ322637.1), two primers were synthesized, whose sequences are shown in Table 1 as P3-nsp4+E3-F1 and P4-E1-R1.
[0142] Table 1. Primers used for the construction of infectious clones of VEEV vaccine strains lacking capsid protein genes
[0143] P3-nsp4+E3-F1 catcgatggcgcgccaccatgtcactagtgaccaccatgtg (SEQ ID NO 2) P4-E1-R1 caatagagtgttctcccac (SEQ ID NO 3)
[0144] The VEEV-delC fragment was amplified by PCR with PrimeSTARMAX enzyme (purchased ...
Embodiment 2
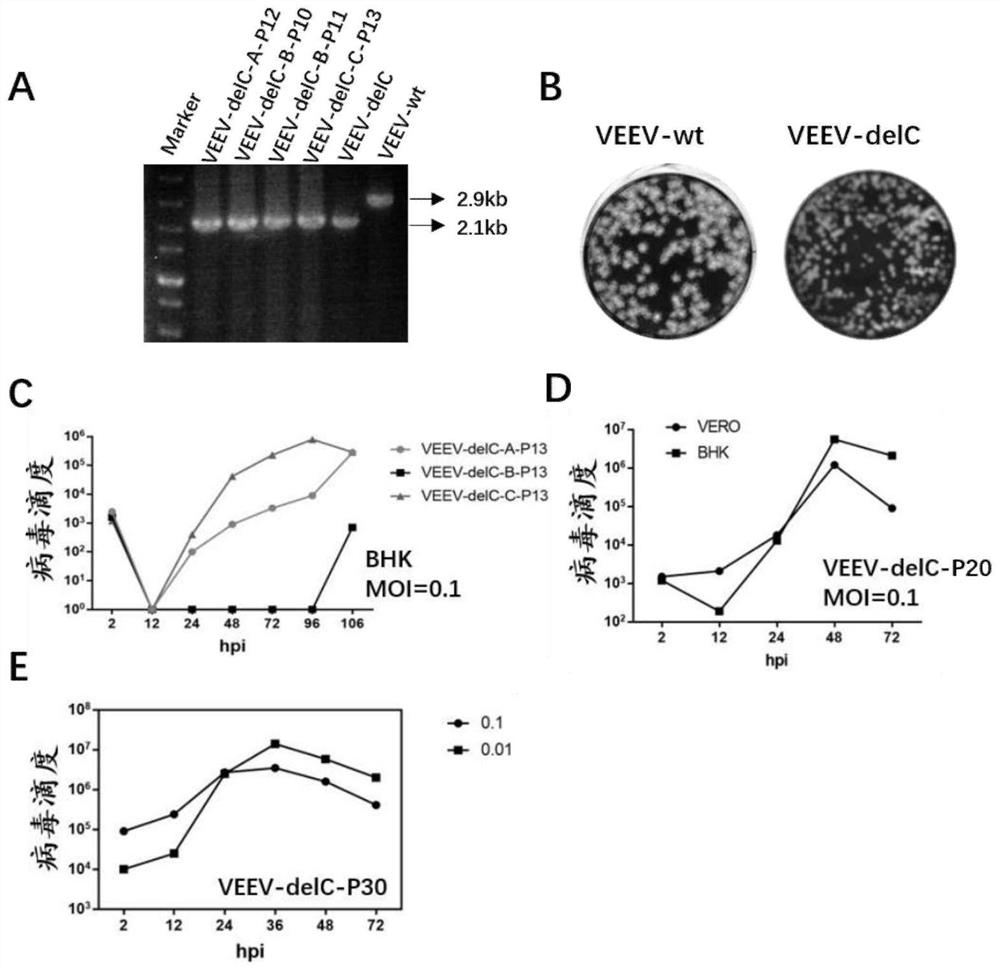
[0160] Example 2: Characteristics of VEEV-delC virus
[0161] The VEEV-delC viruses provided by the present disclosure are genetically stable.
[0162] 2.1. Comparison of VEEV-delC viral plasmid bands passaged for more than 10 generations
[0163] The VEEV-delC virus (P0 generation) rescued in Example 1 was infected with BHK-21 cells, and the cell supernatant (P1 generation) was collected after 5 days, and three viruses were passed in parallel for each generation, and this step was repeated 10 times to obtain the P10 generation. Virus. The P10 generation viruses were divided into three strains, named A strain (VEEV-delC-A-P10), B strain (VEEV-delC-B-P10) and C strain (VEEV-delC-B-P10). The P10 generation virus was further passaged to obtain B strain P11 generation (VEEV-delC-B-P11), A strain P12 generation (VEEV-delC-A-P12) and C strain P13 generation (VEEV-delC-C-P13). RNA was extracted from the cells collected from P0 and P10-P13 passages, and stored at -80°C for future u...
Embodiment 3
[0169] Example 3: Oncolysis of VEEV-delC virus on tumor cells in vitro
[0170] In this example, after infecting tumor cells with the VEEV-delC virus prepared in Example 1 at a fixed MOI dose, the killing effect of VEEV-delC on tumor cells was observed in bright field ( image 3 A), and using CCK8 to detect the killing of tumor cells by different doses of VEEV-delC to determine the in vitro oncolytic effect of the virus ( image 3 B). in:
[0171] 3.1. Bright field observation of the oncolytic effect of VEEV-delC on tumor cells
[0172] Control group (Mock): tumor cell group cultured in medium without virus
[0173] Experimental group: Tumor cell group cultured in medium with VEEV-delC virus
[0174] Eight tumor cells Huh7, A549, SKOV3, B16F1, 4T1, HELA, A375, and A2058 were divided according to 8 × 10 4 Cells / well were seeded in 12-well cell culture plates in DMEM or 1640 medium supplemented with 10% serum (FBS) at 37°C, 5% CO 2 Culture in a humidified incubator. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com