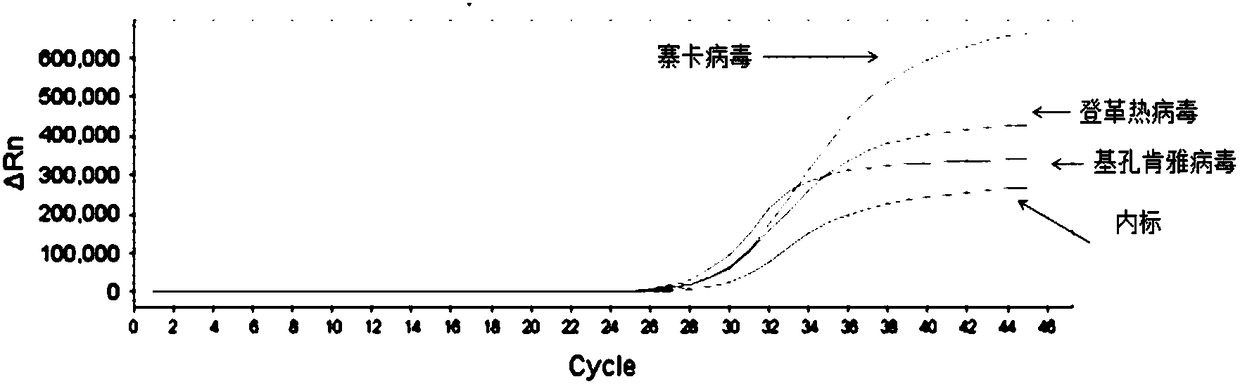
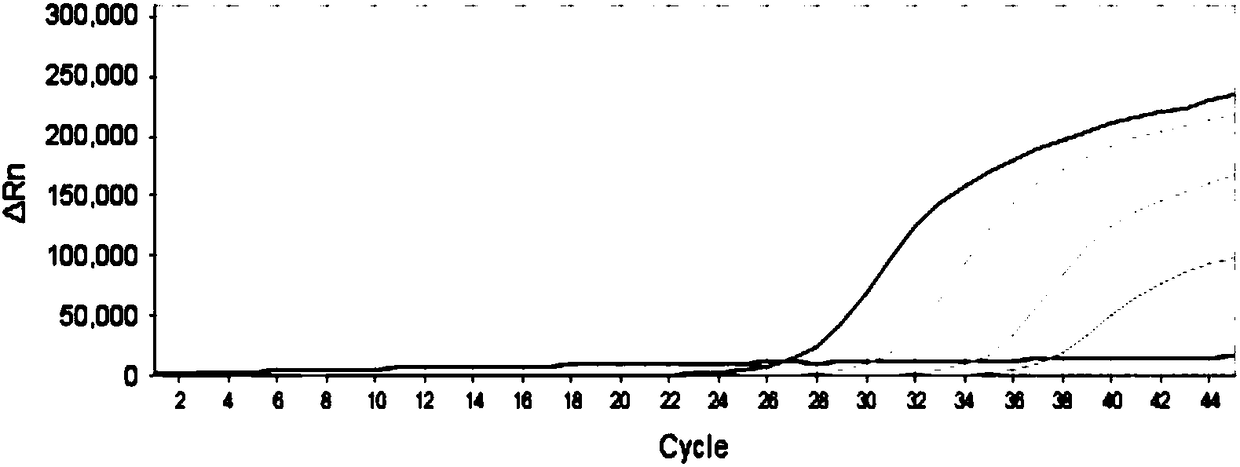
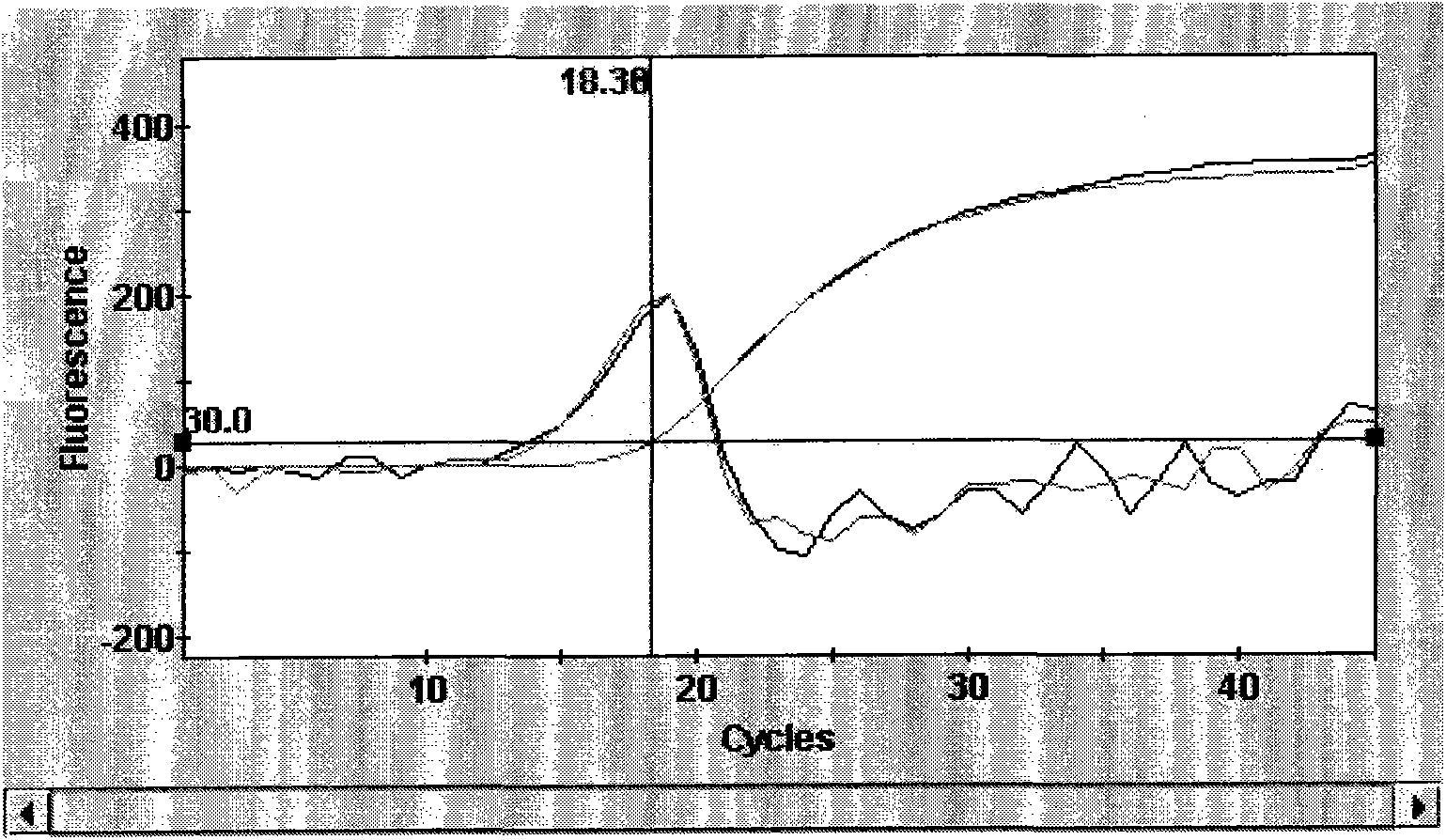
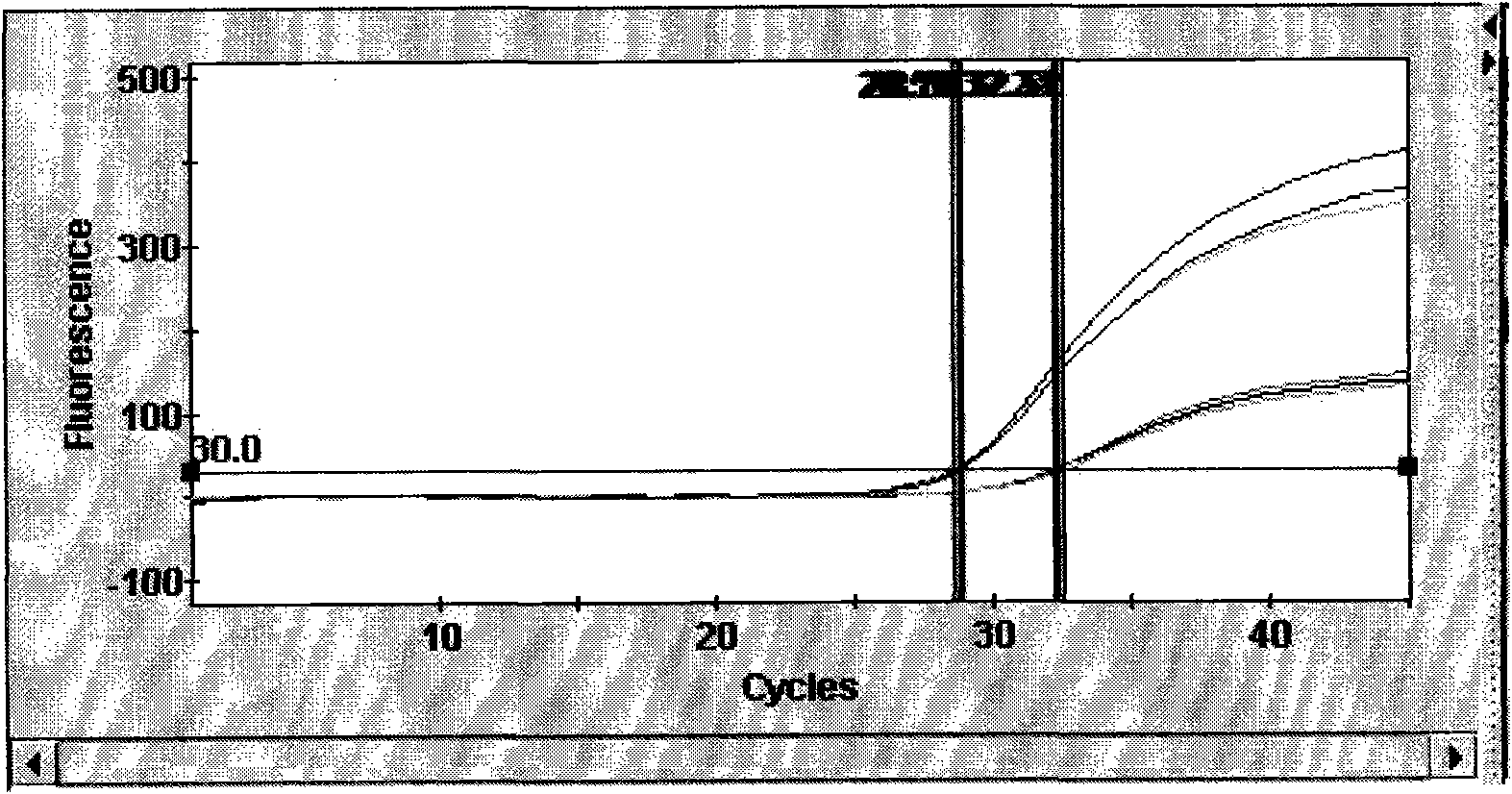
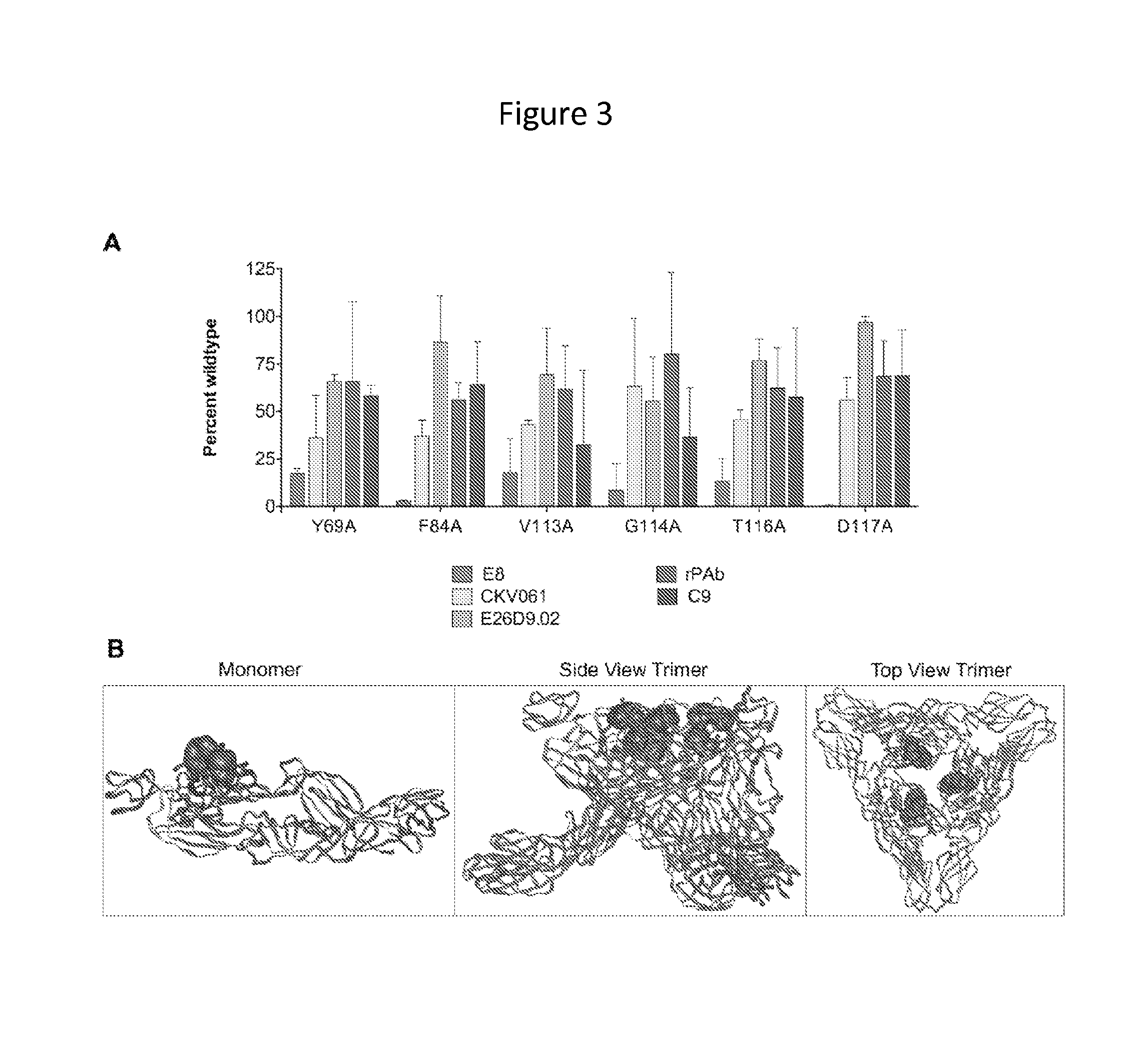
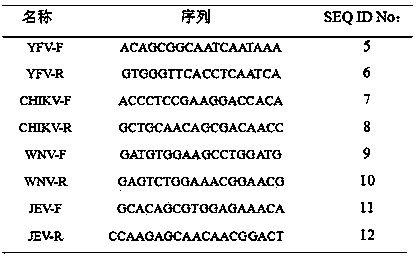
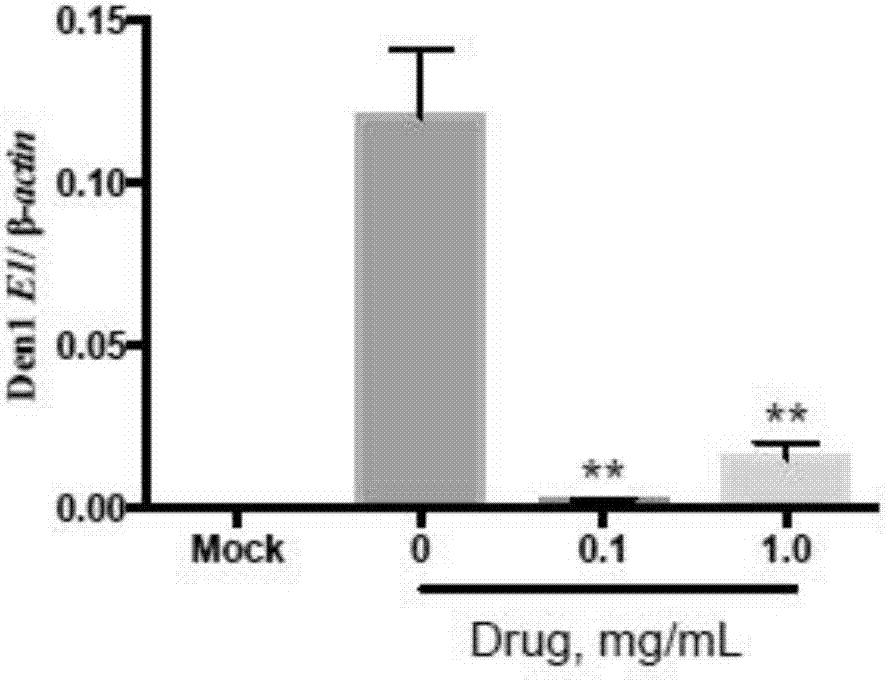
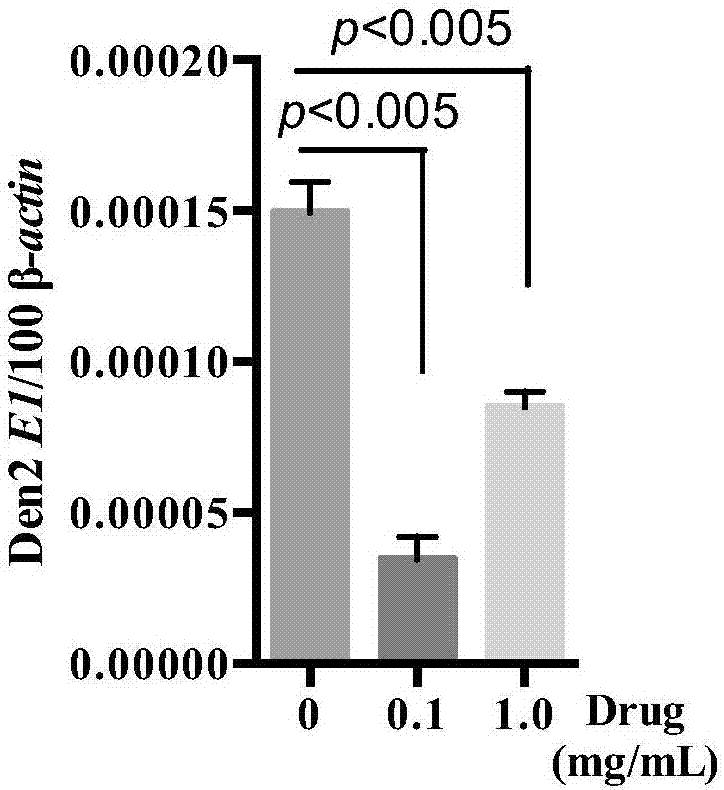
Patents
Literature
104 results about "Chikungunya fever" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Jump to navigation Jump to search. Chikungunya is an infection caused by the chikungunya virus (CHIKV). Symptoms include fever and joint pain. These typically occur two to twelve days after exposure. Other symptoms may include headache, muscle pain, joint swelling, and a rash.
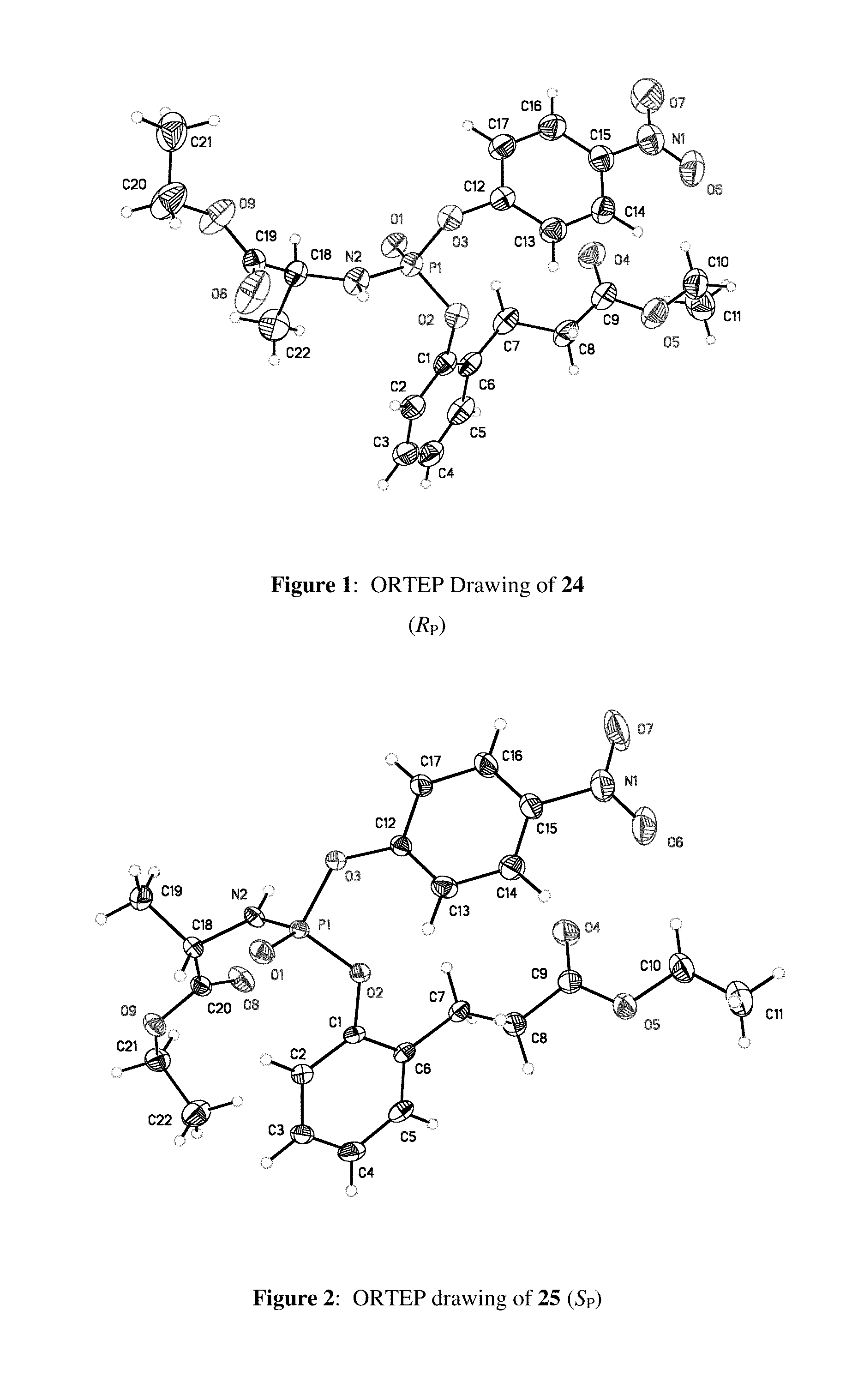
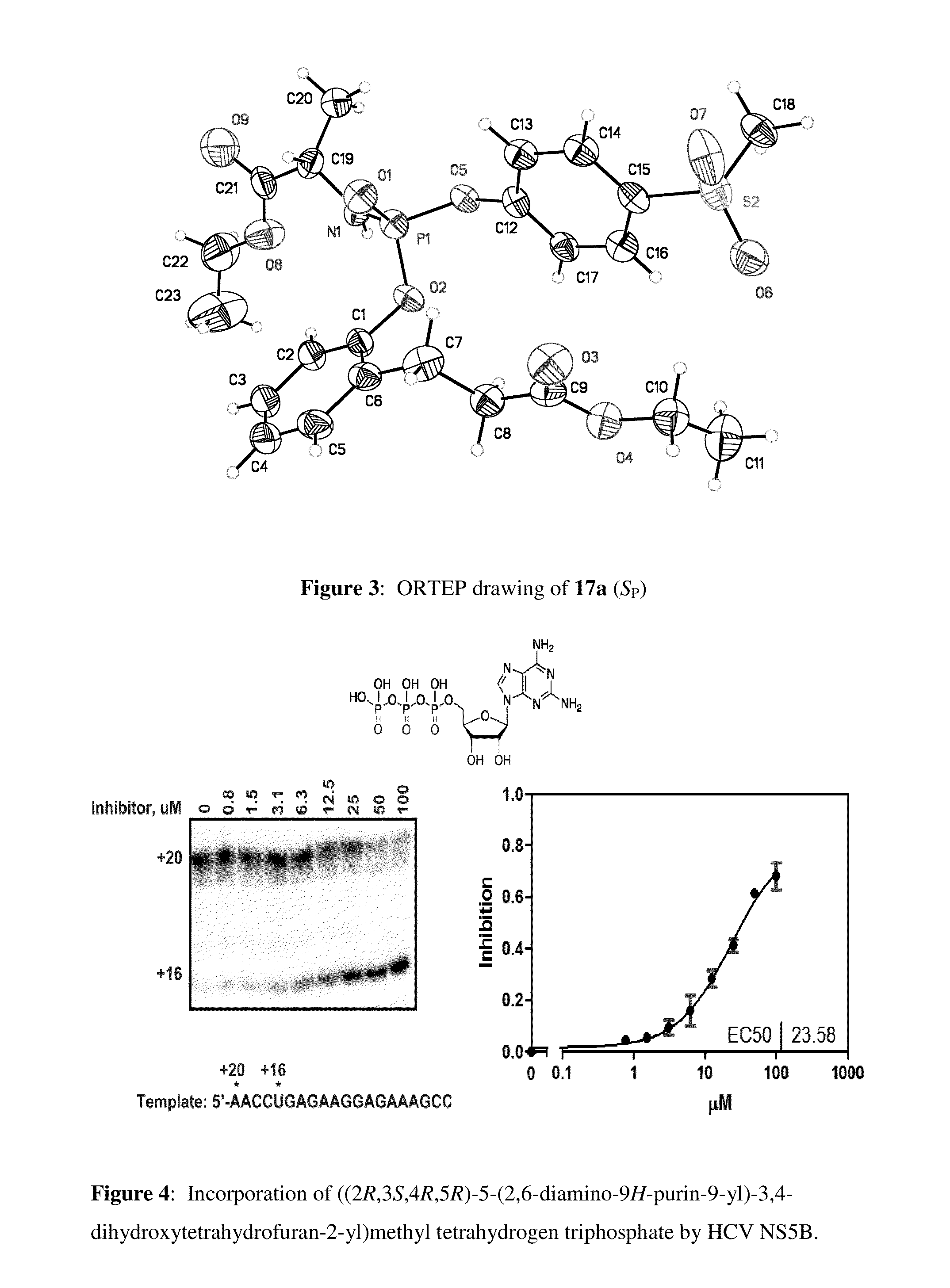
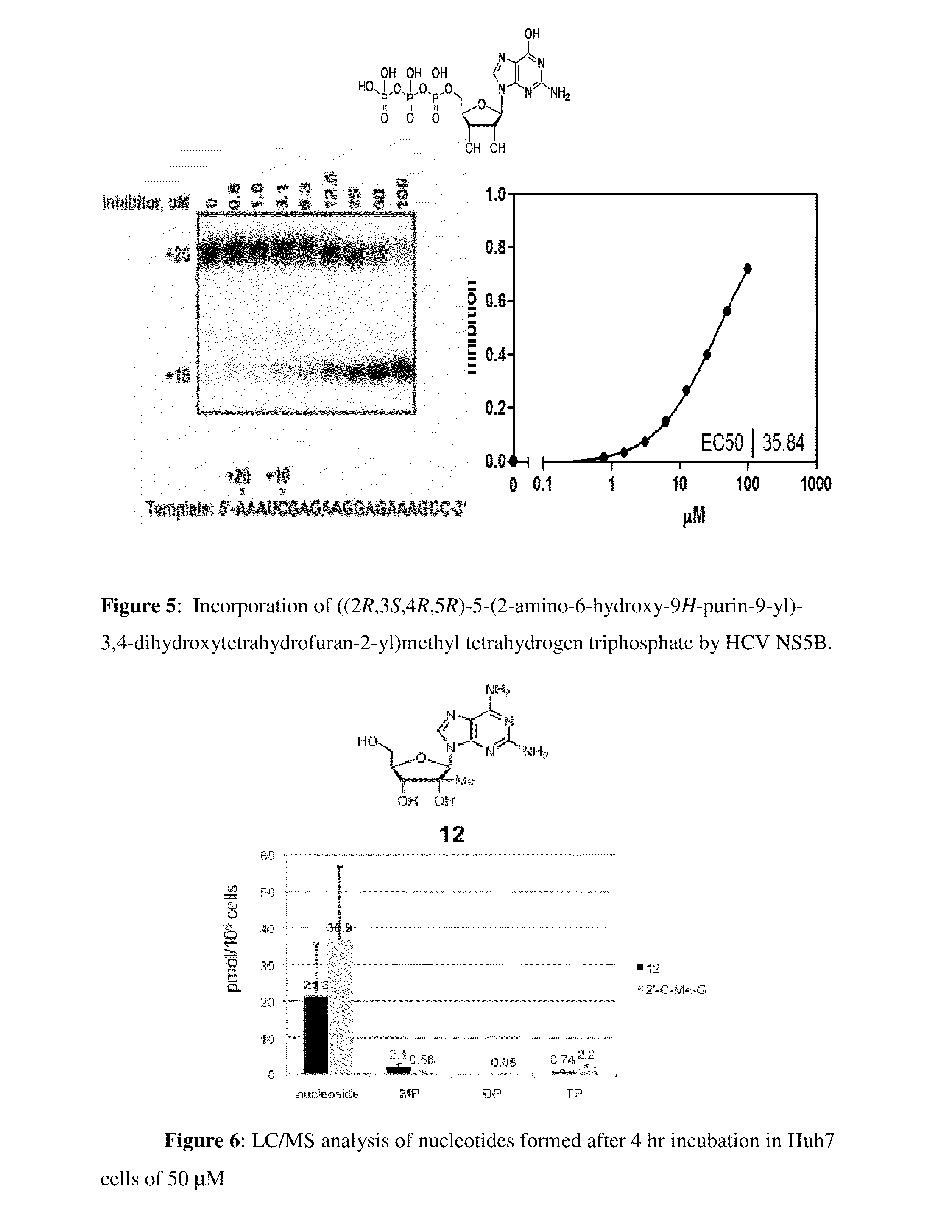
Purine monophosphate prodrugs for treatment of viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing viral infections using nucleoside analog monophosphate prodrugs. More specifically, HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever in human patients or other animal hosts. The compounds are certain 2,6-diamino 2-C-methyl purine nucleoside monophosphate prodrugs and modified prodrug analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever. This invention teaches how to modify the metabolic pathway of 2,6-diamino 2′-C-methyl purine and deliver nucleotide triphosphate(s) to polymerases at heretofore unobtainable therapeutically-relevant concentrations.
Owner:COCRYSTAL PHARMA INC
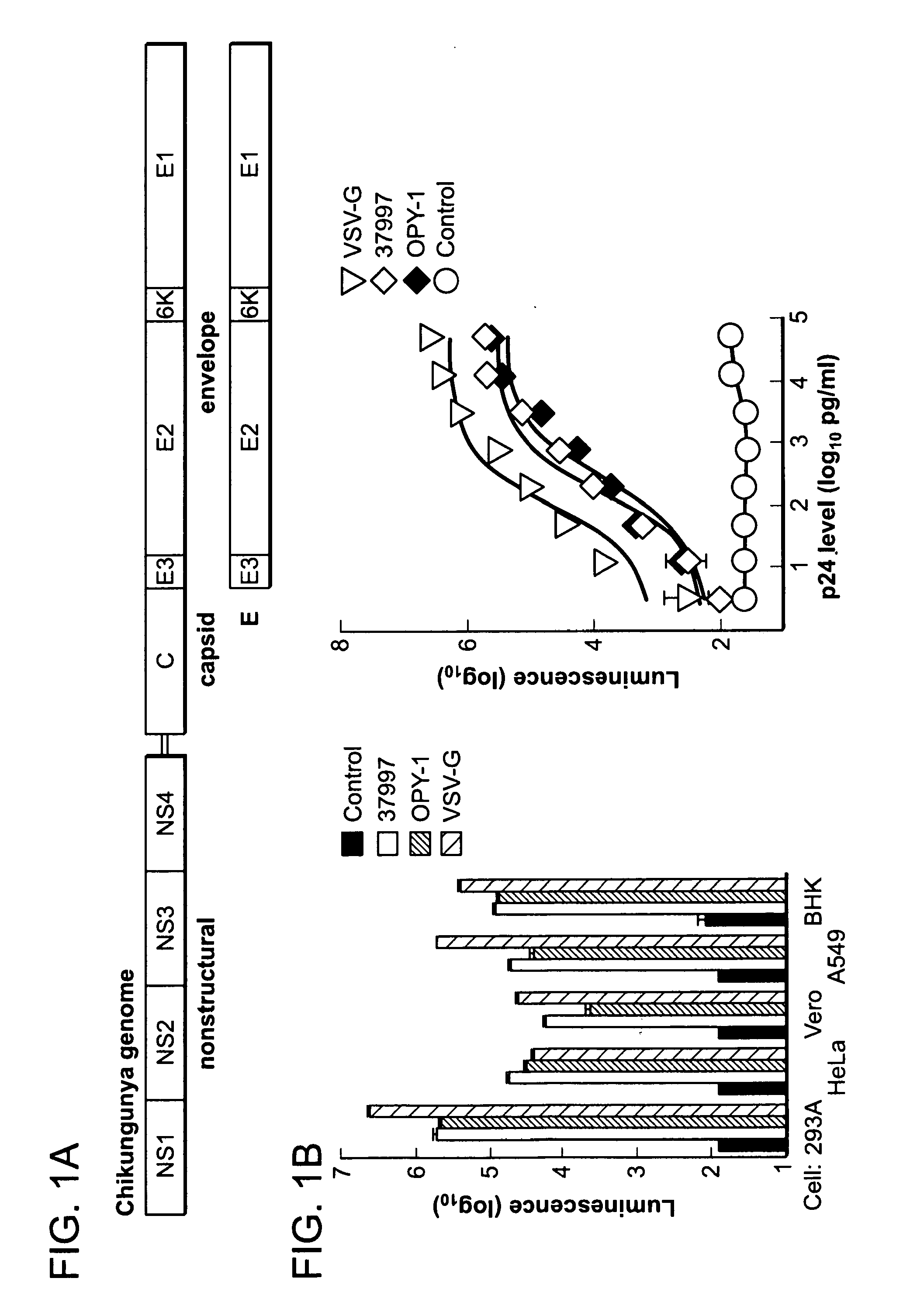
Virus like particle compositions and methods of use
ActiveUS20120003266A1Improve purification effectAvoid infectionFungiSsRNA viruses positive-senseVirus-like particleChikungunya fever
The invention features compositions and methods for the prevention or treatment of one or more strains of Chikungunya virus, as well as other alphavirus-mediated diseases.
Owner:UNITED STATES OF AMERICA
Preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV) and its application
InactiveCN102321639ANeutralizing activityViral antigen ingredientsInactivation/attenuationImmune effectsVirus-like particle
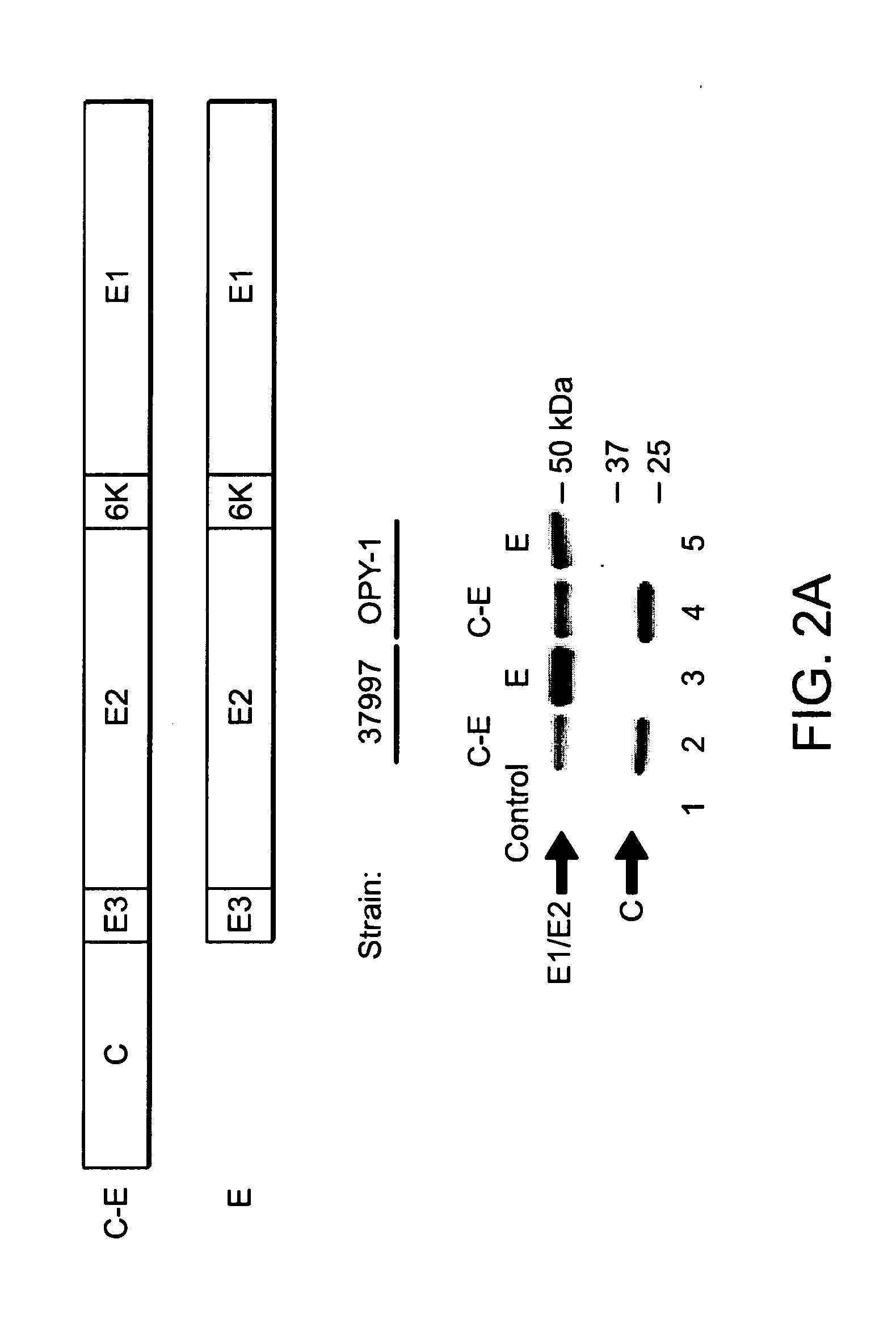
The invention relates to a preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV). The method comprises the steps of: modifying genetic elements of a structural protein encoding gene C-E3-E2-6K-E1 of CHIKV, cloning the modified genetic elements into the expression vector of an insect cell, then transfecting the obtained recombined expression vector and baculovirus linear DNA respectively to an SF9 insect cell and making the cell secrete and express CHIKV VLPs. Additionally, the invention also makes preliminary studies on the immune effects of CHIKV VLPs and applicationof CHIKV VLPs in virus specific antibody detection, thus laying a foundation for research and preparation of immunological detection reagents and even vaccines based on CHIKV VLPs.
Owner:中国疾病预防控制中心病毒病预防控制所
Virus-like particles (VLPs) prepared from chikungunya virus structural proteins
ActiveUS9353353B2Avoid infectionRelieve symptomsSsRNA viruses positive-senseAntipyreticDiseaseVirus-like particle
The invention features compositions and methods for the prevention or treatment of one or more strains of Chikungunya virus, as well as other alphavirus-mediated diseases.
Owner:UNITED STATES OF AMERICA
Binding Molecules Against Chikungunya Virus and Uses Thereof
ActiveUS20130189279A1Avoid reactionAvoid secondary effectsBacteriaPeptide/protein ingredientsChikungunya feverResearch purpose
The invention relates to binding molecules against Chikungunya virus, which are able of neutralizing Chikungunya virus infectivity, and which can be used with therapeutic, diagnosis or research purposes, as well as to a pharmaceutical composition comprising said binding molecules.
Owner:AGENCY FOR SCI TECH & RES
Chikungunya virus testing method
InactiveCN101270394AEnhanced protection against incomingMicrobiological testing/measurementAgainst vector-borne diseasesRNA extractionFluorescence
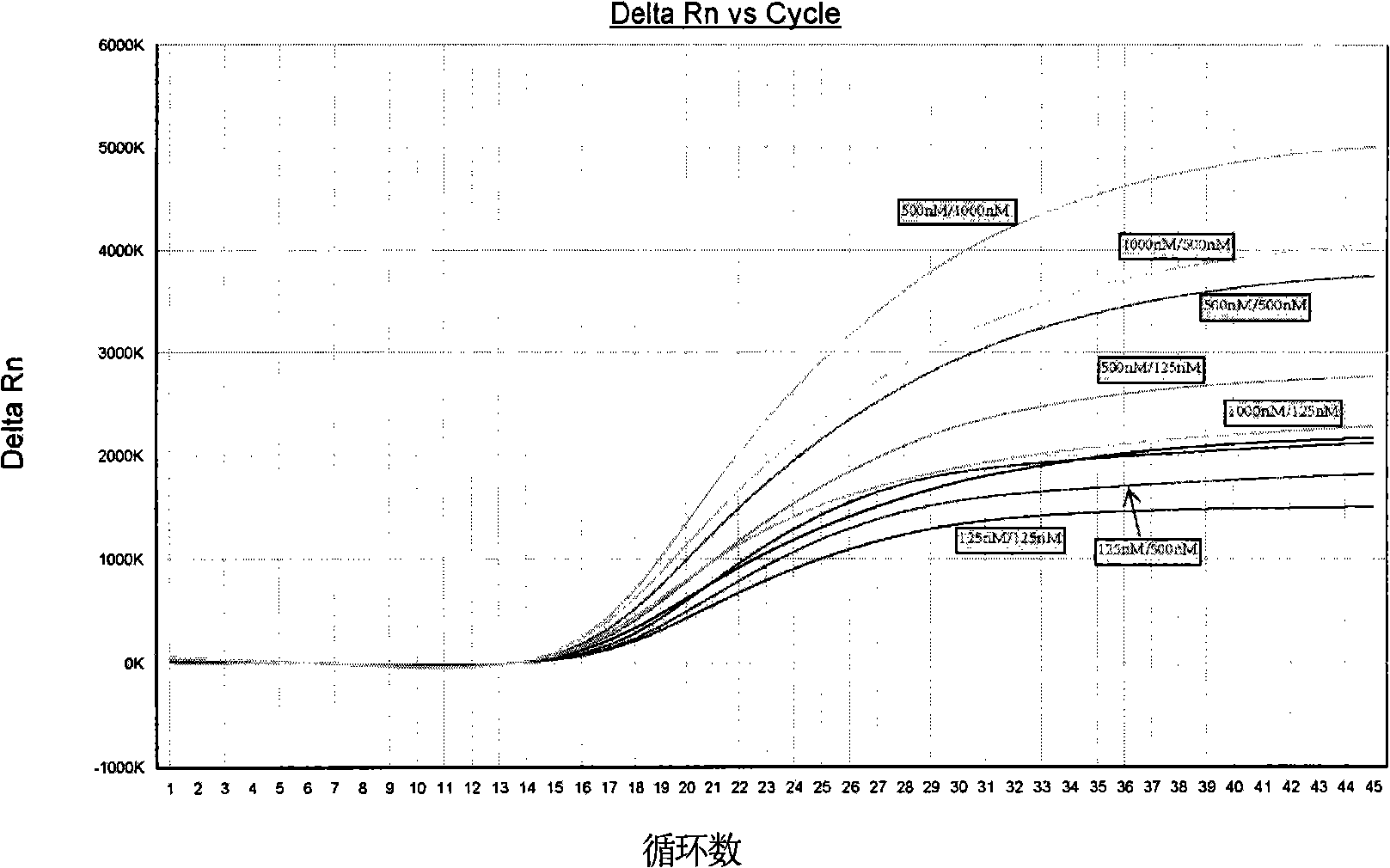
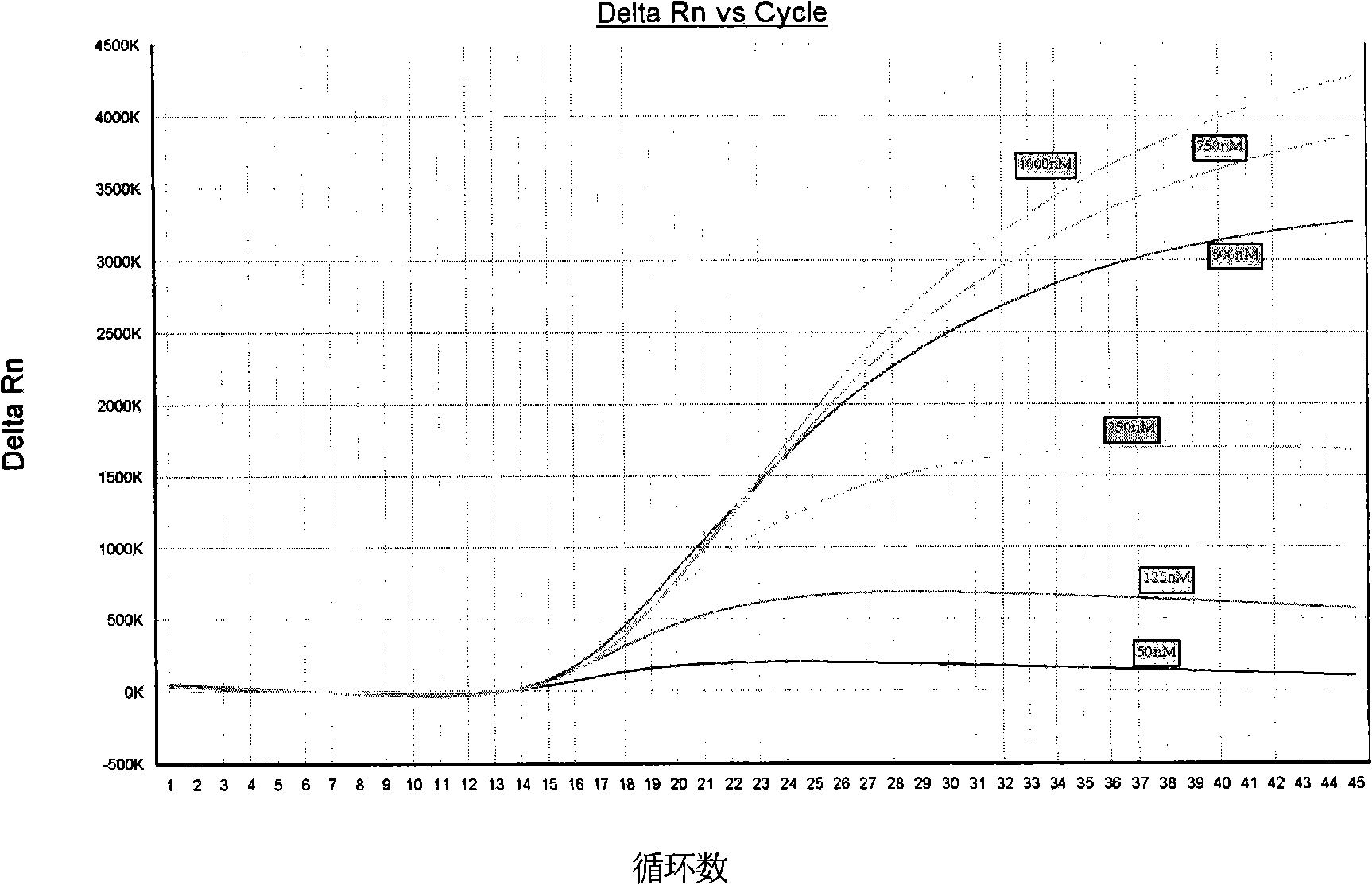
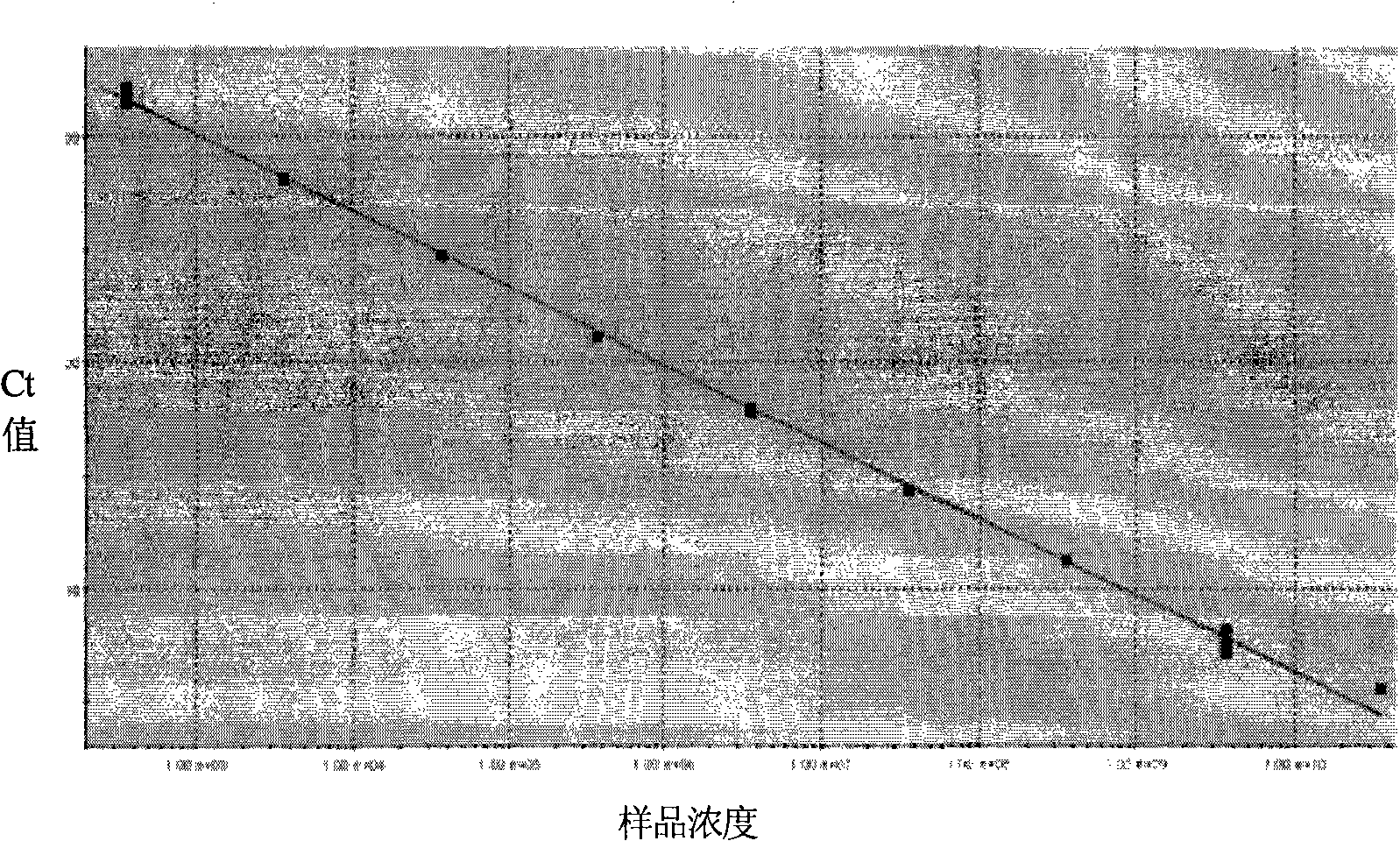
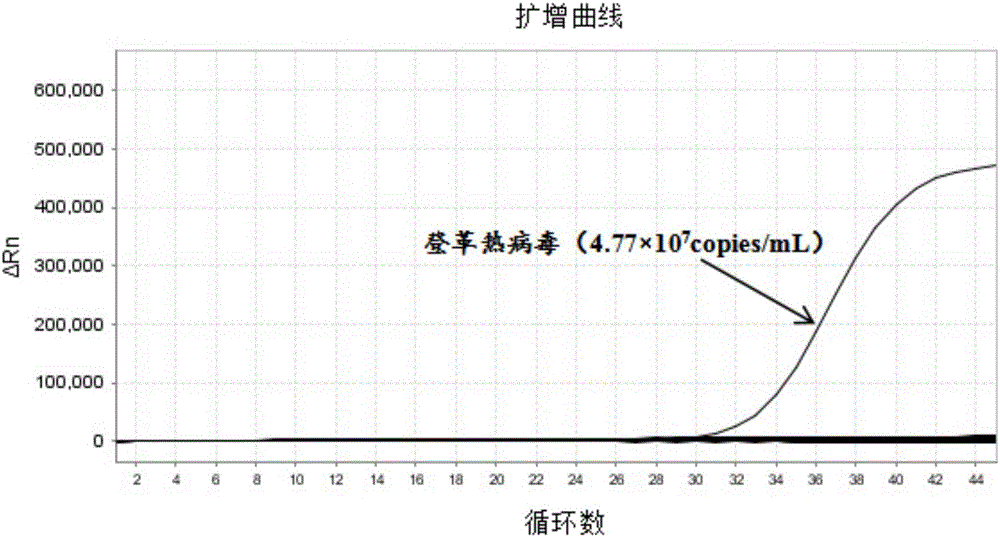
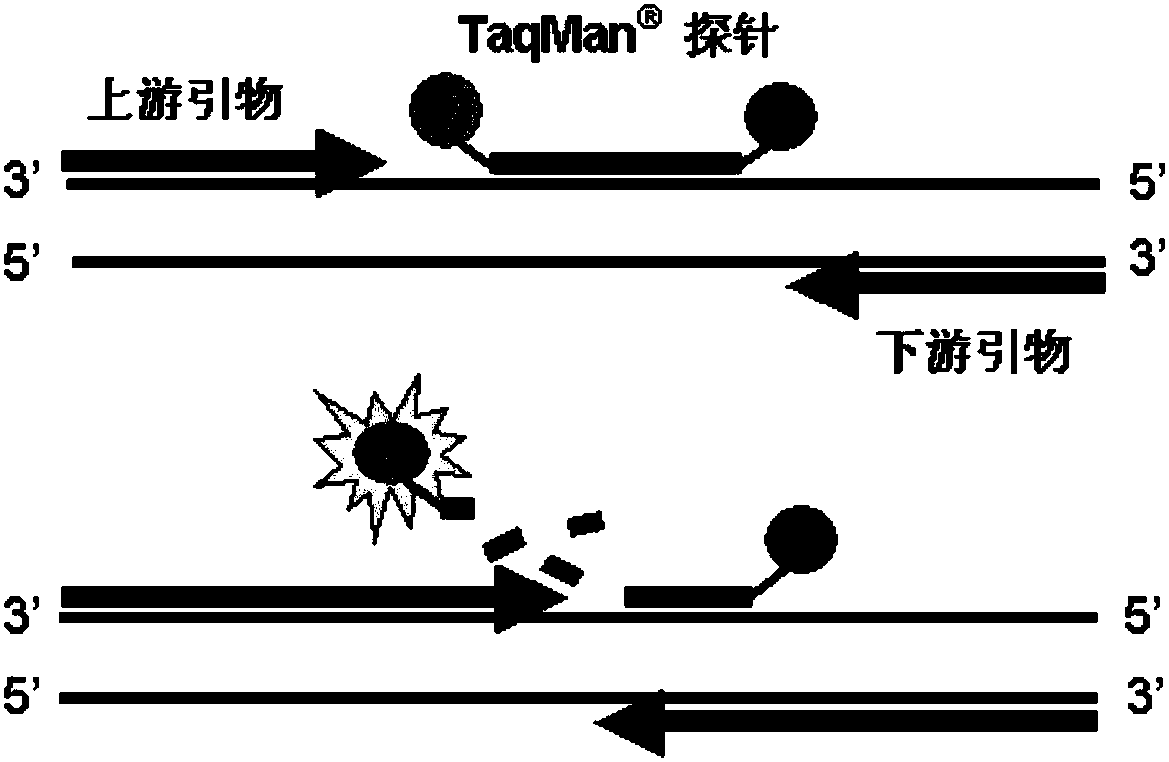
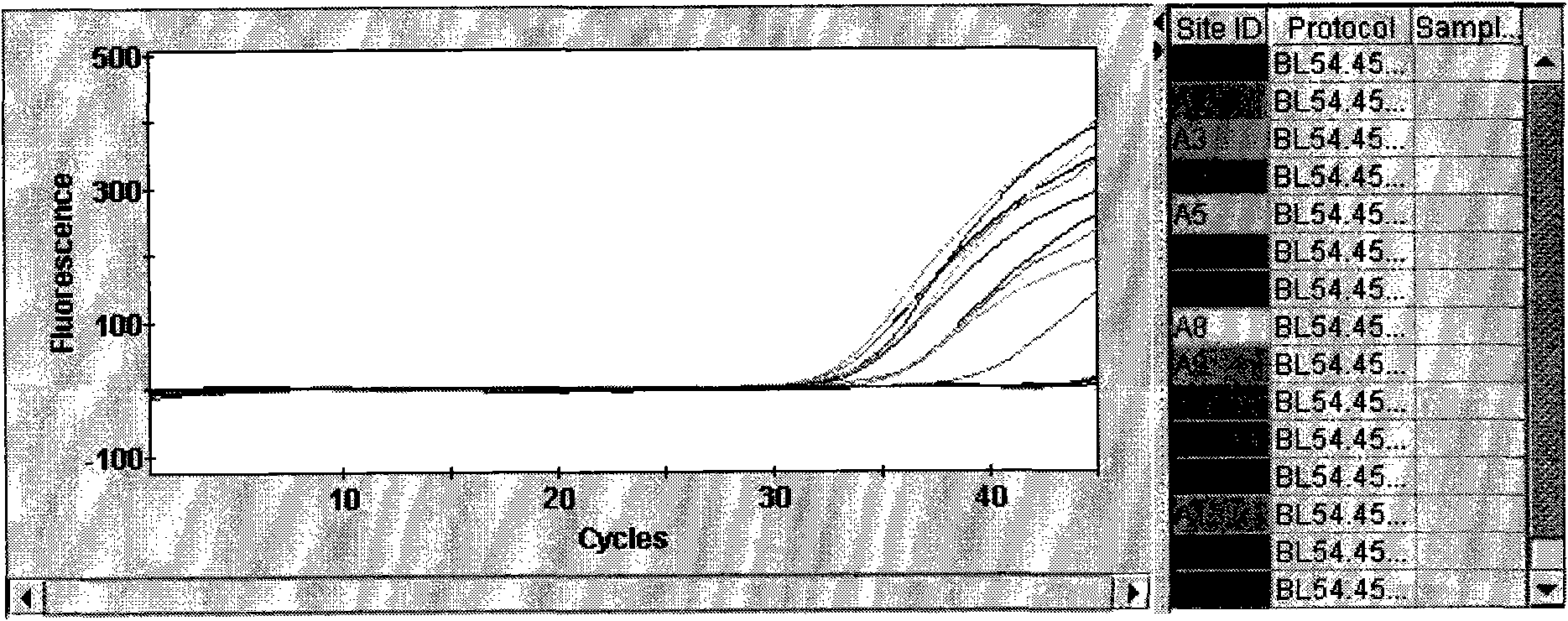
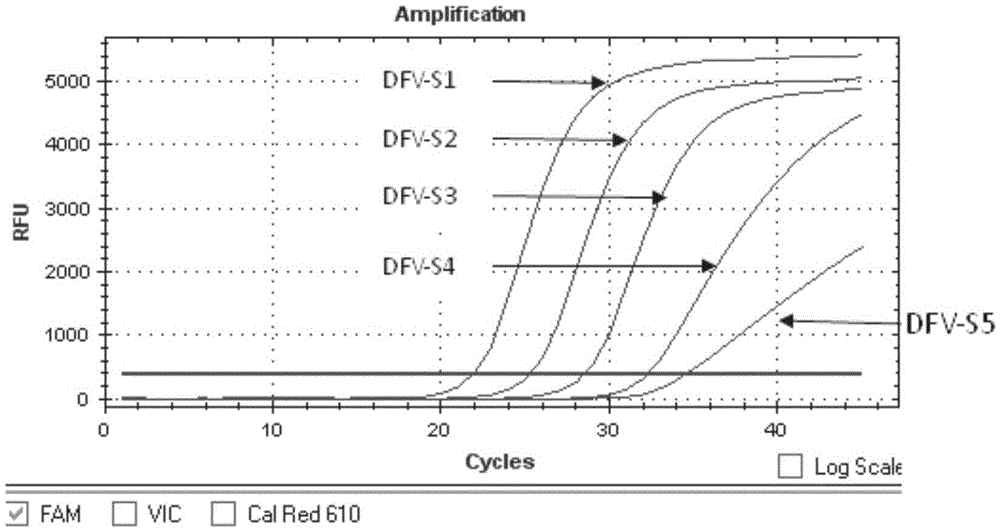
The invention provides a detection method of Chikungunya virus, which comprises the procedures as follows: pretreatment of test sample; RNA extraction of test sample to be detected; design and synthesis of primer and probe; determination of optimum fluorescence quantitative PCR reaction system; program amplification and adjudging results according to Ct value. The optimum fluorescence quantitative PCR reaction system is determined as follows: the CHIK-FP terminal concentration of positive primer is 500nM, the CHIK-RP terminal concentration of reverse primer is 1000nM, and the CHIK-Probe terminal concentration of probe is 250nM. The amplification program is 45 times of circulation of 10 minutes at 50 DEG C, 10 minutes at 95 DEG C, 10 seconds at 95 DEG C and 30 seconds at 62 DEG C. The invention establishes a detection method for Chikungunya virus, which can be applied on monitoring of the virus, and can further strengthen the monitoring work for preventing from spreading the disease into our country and has important social efficiency and economic efficiency.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS10072309B1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaFluorescence
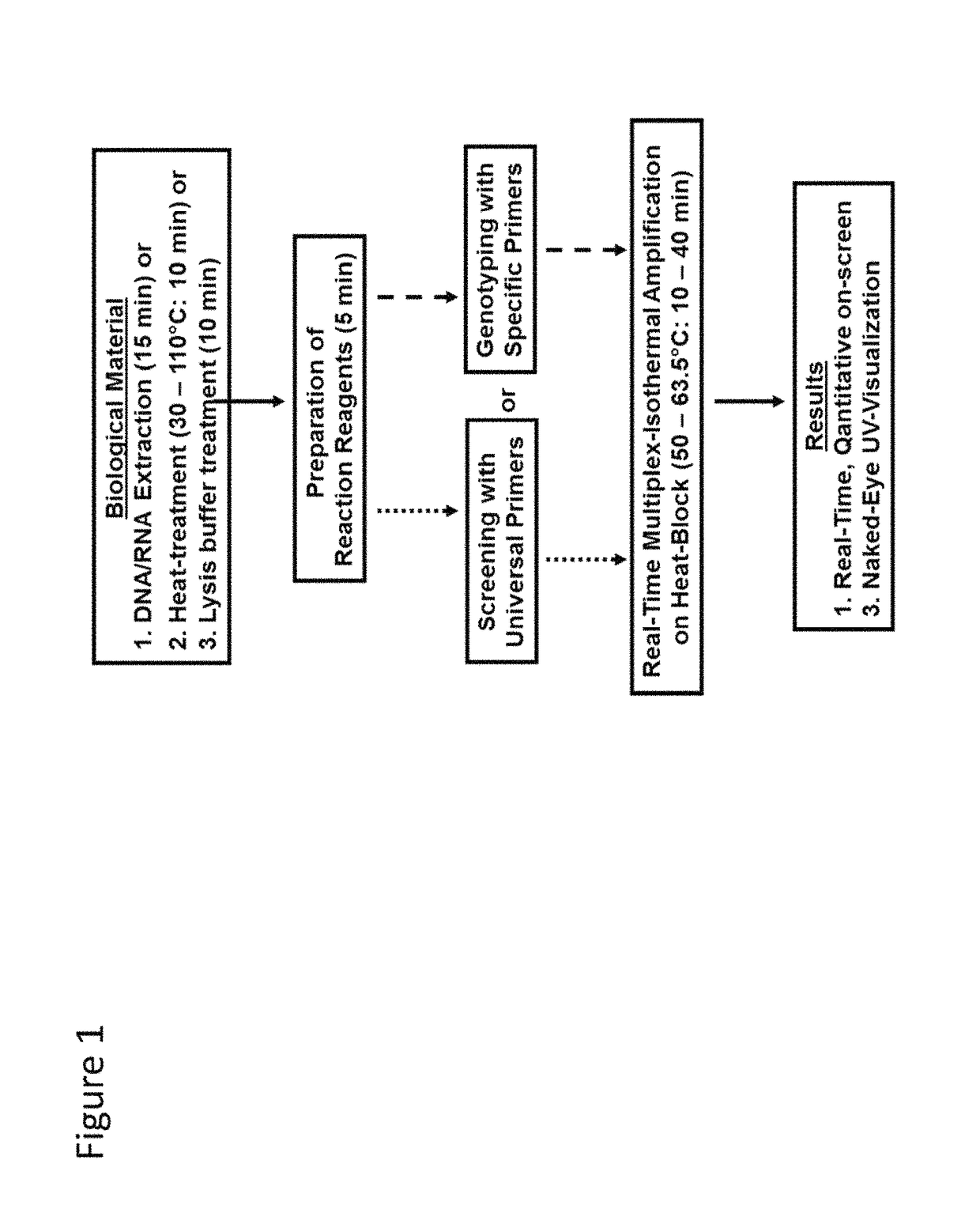
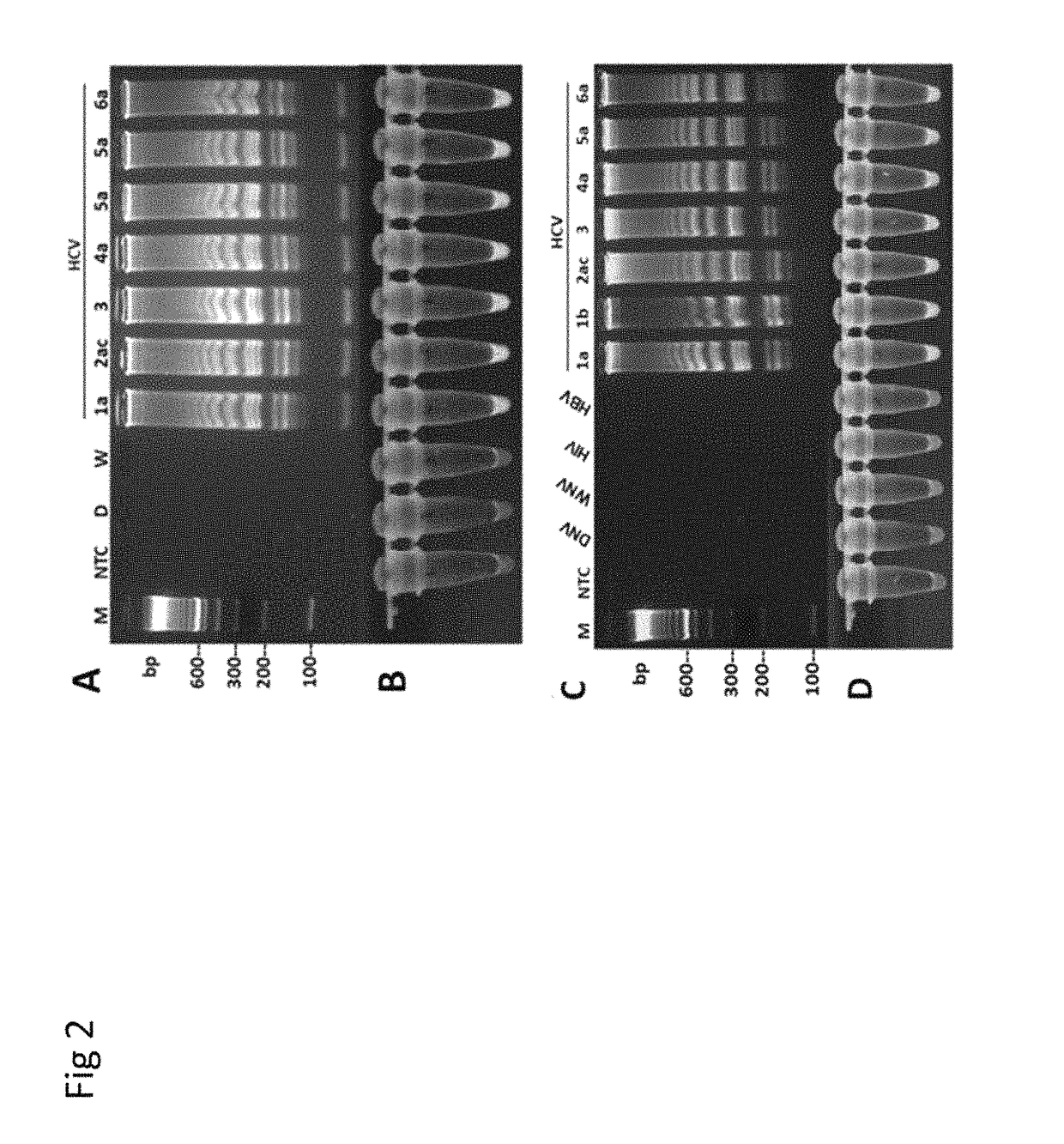
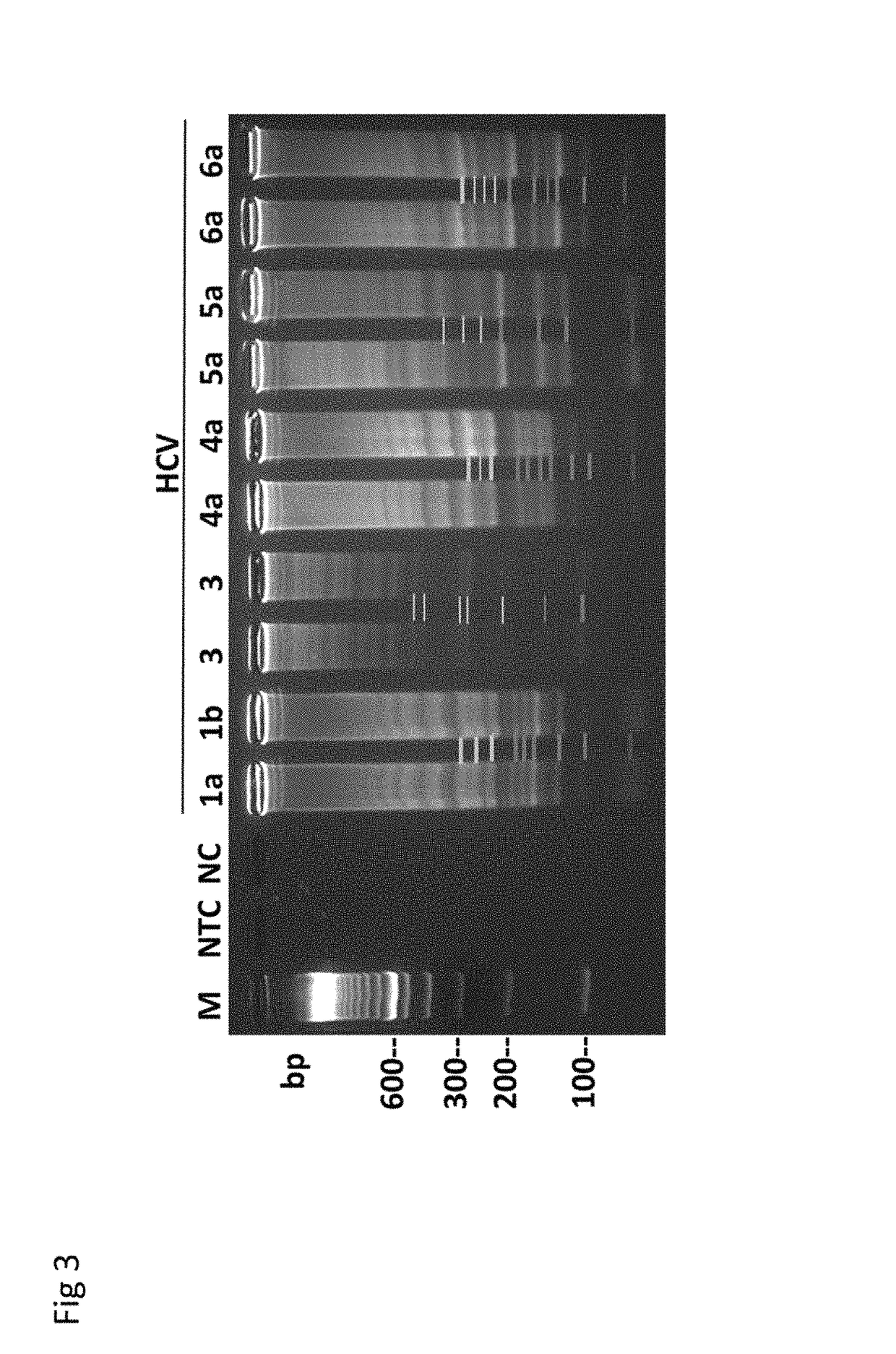
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Kit for detecting mosquito borne pathogens and detection method thereof
InactiveCN103911463AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesEpidemic encephalitisMultiplex polymerase chain reaction
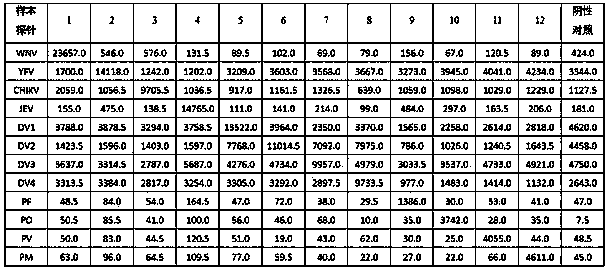
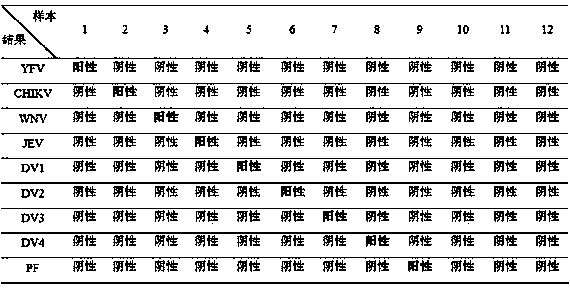
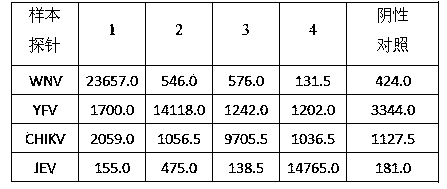
The invention discloses a kit for detecting a plurality of mosquito borne pathogens and a detection method thereof. The plurality of mosquito-borne infectious pathogens are detected by using multiplex polymerase chain reaction (PCR) combined with a liquid chip, and a yellow fever virus (YFV), dengue fever viruses (DV) I-IV type, epidemic encephalitis B (japanese encephalitis) virus (JEV), plasmodium (Plasmodium), (including plasmodium vivax (PV), plasmodium falciparum (PF), plasmodium malariae (PM), plasmodium ovale (PO)), a west nile virus (MNV) and a chikungunya virus (CHIKV) can be detected in parallel one time. The kit has the advantages of being large in flux, high in specificity and sensitivity, stable in result, good in repeatability, simple to operate and fast in detection speed.
Owner:河北国际旅行卫生保健中心
Attenuated recombinant alphaviruses incapable of replicating in mosquitoes and uses thereof
ActiveUS20110052634A1SsRNA viruses positive-senseMutant preparationEastern equine encephalitis virusEncephalitis Viruses
The present invention discloses an attenuated recombinant alphavirus that is incapable of replicating in mosquito cells and of transmission by mosquito vectors. These attenuated alphavirus may include but is not limited to Western Equine Encephalitis virus, Eastern equine encephalitis virus, Venezuelan equine encephalitis virus or Chikungunya virus. The present invention also discloses the method of generating such alphaviruses and their use as immunogenic compositions.
Kit for flavivirus quick typing and virus load detection
InactiveCN106086242ANo cross reactionAchieve quantitative goalsMicrobiological testing/measurementAgainst vector-borne diseasesZika virusMicroorganism
The invention belongs to the field of microbial molecular detection, and particularly relates to a kit for flavivirus quick typing and virus load detection. The kit for flavivirus quick typing and virus load detection comprises specific primers and probes for flaviviruses, including specific primers and a probe for dengue virus, specific primers and a probe for Zika virus, specific primers and a probe for yellow fever virus, and specific primers and a probe for Chikungunya virus. The kit for flavivirus quick typing and virus load detection has the advantages of high detection speed and accurate detection and quantification results, can simultaneously detect 3 different flavivirus pathogens and 1 togavirus pathogen capable of causing similar clinical symptoms at one time, can detect and diagnose flavivirus-pathogen-infected suspicious cases in time, and enhances the detection accuracy of flavivirus pathogen infection.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL
RT-PCR detection method, primer and probe as well as kit of Zika virus, dengue virus and chikungunya virus
InactiveCN106755573AQuick checkAccurate detectionMicrobiological testing/measurementMicroorganism based processesZika virusFluorescence
The invention relates to the technical field of molecular biological detection of an insect-borne infectious disease, and discloses a triple real-time fluorescence RT-PCR detection method, primer and probe as well as kit of Zika virus, dengue virus and chikungunya virus. The primer comprises the sequences of SEQ ID No.1, SEQ ID No.2, SEQ ID No.4, SEQ ID No.5, SEQ ID No.7 and SEQ ID No.8; the probe comprises the sequences of SEQ ID No.3, SEQ ID No.6, and SEQ ID No.9; the kit comprises a primer probe mixed solution, an RT-PCR reaction solution, an enzyme mixed solution, and a positive standard substance. The Zika virus, the dengue virus and the chikungunya virus can be fast detected in the reaction solution of a same tube by use of a triple real-time fluorescence RT-PCR amplification technology by optimizing the reaction solution formula and the primer probe sequences, the single detection is unnecessary; compared with the traditional PCR detection method, the operation step is reduced, whether the reaction solution contains the three virus can be fast and accurately detected, the detection time is shortened, the detection efficiency is improved, and the cost is saved.
Owner:深圳澳东检验检测科技有限公司
Nucleic acid detection kit for Zika virus, dengue fever virus and Chikungunya virus, and application of the kit
ActiveCN108330210ASolve the problem of not being able to detect three viruses simultaneously in one reactionSimple and fast operationMicrobiological testing/measurementAgainst vector-borne diseasesZika virusPositive control
The invention discloses a nucleic acid detection kit for Zika virus, dengue fever virus and Chikungunya virus, and an application of the kit, wherein the kit includes: a RT-PCR reaction solution, an enzyme mixture liquid, a Zika virus / dengue fever virus / Chikungunya virus / endogenous reference gene quadruple reaction solution, a positive control, and a blank control. The kit can simultaneously detect the Zika virus, dengue fever virus and Chikungunya virus in one reaction and solves a problem that an in-vitro detection kit cannot simultaneously detect the Zika virus, dengue fever virus and Chikungunya virus in one reaction tube; besides, the detection kit is high in specificity and sensitivity, has simple operation, excellent repeatability and quick and objection detection results. The invention provides an effective technical means for in-vitro detection of the Zika virus, dengue fever virus and Chikungunya virus.
Owner:GUANGZHOU CENT FOR DISEASE CONTROL & PREVENTION (GUANGZHOU HYGIENE INSPECTION CENT GUANGZHOU CENT FOR FOOD SAFETY RISK SURVEILLANCE & ASSESSMENT INST OF PUBLIC HEALTH OF GUANGZHOU MEDICAL UNIV) +1
New chikungunya virus fluorescence quantitative polymerase chain reaction detection method and chikungunya virus polymerase chain reaction detection system
ActiveCN102140530AStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceChikungunya fever
The invention discloses a new chikungunya virus fluorescence quantitative polymerase chain reaction (PCR) detection method and a chikungunya virus PCR detection system. The detection system consists of a primer and a probe, Premix Ex Taq reaction solution and sterilized Tris solution, wherein the primer and probe have good detection specificity. The system has high sensitivity, is very suitable for chikungunya virus and does not have cross reactions with the dengue virus and the like.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Concentrate of Chikungunya-Specific Immunoglobulins as a Medicinal Product
The invention concerns a new medicinal product for the treatment of chikungunya, i.e a concentrate of chikungunya-specific immunoglobulins, as well as its process of preparation.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
4'-halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto
Disclosed are halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto. In certain embodiments, the disclosure relates to the treatment or prophylaxis of viral infections. Such viral infections can include tongaviridae, bunyaviridae, arenaviridae, coronaviridae, flaviviridae, picornaviridae, Eastern, Western, and Venezuelan Equine Encephalitis (EEE, WEE and VEE, respectively), Chikungunya fever (CHIK), Ebola, Influenza, RSV, and Zika virus infections.
Owner:EMORY UNIVERSITY
Chimeric chikungunya virus and uses thereof
ActiveUS20110171249A1Avoid infectionSsRNA viruses positive-senseSugar derivativesSindbis virusChikungunya fever
The present invention discloses a chimeric Chikungunya virus comprising a heterologous alphavirus cDNA fragment and a Chikungunya virus cDNA fragment. The heterologous alphavirus may include but is not limited to Sindbis virus, Eastern equine encephalitis virus or Venezuelan equine encephalitis virus. The present invention also discloses the use of this chimeric Chikungunya virus as vaccines and in serological and diagnostic assays.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for detecting Zika virus, Chikungunya virus and Mayaro virus by triple real-time fluorescent quantitative RT-PCR
ActiveCN110305985AImprove universalityStrong specificityMicrobiological testing/measurementMicroorganism based processesZika virusChikungunya fever
The invention relates to the technical field of virus detection, and concretely relates to a method for detecting Zika virus, Chikungunya virus and Mayaro virus by triple real-time fluorescent quantitative RT-PCR. The method combines the probability of occurrence of related mosquito-borne pathogens, the risk of prevalence, and the operability of a laboratory testing procedure, the Mayaro virus isused to replace the dengue virus in an existing common combination detection scheme, and a new detection scheme using Zika virus, Chikungunya virus and Mayaro virus as detection targets is formed. Theinvention provides a specific primer, a probe and a triple real-time quantitative RT-PCR detection method for simultaneously identifying the viral pathogens Zika virus, Chikungunya virus and Mayaro virus which are transmitted by Aedes mosquito and cause similar clinical symptoms of diseases. The method has high specificity and sensitivity, good repeatability, simple and rapid detection, and costsaving, and can complement a traditional detection scheme and has high application value.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Antibodies against chikungunya virus and uses thereof
ActiveUS20160145323A1Reduces PathologySsRNA viruses positive-senseImmunoglobulins against virusesHeterologousEpitope
Embodiments disclosed herein provide for antibodies, including neutralizing antibodies, against Chikungunya virus, uses thereof, and methods of identifying antibodies, including neutralizing antibodies, against Chikungunya virus. In some embodiments, antibodies that binds to a CHIKV antigen, wherein the antigen is the CHIKV E1, E2, E3 protein, or any heterocomplex thereof are provided. In some embodiments, the antibody is an isolated antibody, a neutralizing antibody, a recombinant antibody, or any combination thereof. In some embodiments, the antibodies described herein bind to an epitope of E2 Domain A, E2 Domain B, or E1 Domain II of the CHIKV antigen. In some embodiments, the antigen is E2 protein.
Owner:BLOOD SYST RES INST +1
Preparation and application of hemorrhagic fever associated pathogen identifying gene chip
ActiveCN105087824APracticalShort detection cycleNucleotide librariesMicrobiological testing/measurementOligonucleotide chipOligonucleotide
The invention relates to a hemorrhagic fever associated pathogen identifying gene chip; the preparation method comprises preparation of a specific primer, preparation of a pathogen specific oligonucleotide probe, preparation of an oligonucleotide chip, establishment of an RT-PCR (reverse transcription-polymerase chain reaction) system and establishment of a hybrid system and a signal detection method. The gene chip prepared by the invention can be used for simultaneously identifying 16 hemorrhagic fever associated pathogen microorganisms, including Zaire Ebola virus, Sudan Ebola virus, marburg virus, lassa virus, junin virus, Machupo virus, rift valley fever virus, Crimea-Congo hemorrhagic fever virus, plasmodium, hantaan virus, SFTS (severe fever with thrombocytopenia syndrome) virus, dengue virus, yellow fever virus, Chikungunya virus, influenza A virus and influenza B virus. The gene chip has the characteristics of being rapid and accurate, high in throughput and high in sensitivity; and a new technological means is offered for the diagnosis of hemorrhagic fever pathogen, health supervision and the control and prevention of infectious diseases.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION +1
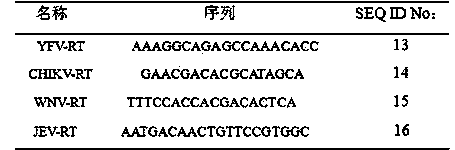
Identification and detection method for yellow fever, Japanese encephalitis, chikungunya fever and west Nile fever, primers and probes
InactiveCN103911462AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesChikungunya feverRepeatability
The invention discloses an identification and detection method for yellow fever, Japanese encephalitis, chikungunya fever and west Nile fever, primers and probes. The identification and detection method comprises the step of rapidly, qualitatively and quantitatively detecting a yellow fever virus, a Japanese encephalitis virus, a chikungunya virus and a west Nile virus by combining polymerase chain reaction (PCR) and a liquid chip. The four pairs of primers and probes with high specificity, high sensitivity and good repeatability are provided in the method.
Owner:河北国际旅行卫生保健中心
Fulminating-infectious-disease pathogen detecting primer pair and kit
ActiveCN105483293AReduced Pollution ChancesShorten detection timeMicrobiological testing/measurementAgainst vector-borne diseasesColor changesBiology
The invention discloses a fulminating-infectious-disease pathogen detecting primer pair and a kit. The primer pair comprises at least a pair of RT-LAMP primers of Ebola viruses, Lassa fever viruses, Marburg viruses, rift valley fever viruses, yellow fever viruses and Chikungunya fever viruses. By means of the primer system, the amplification reaction background is reduced, and sensitivity and specificity are quite good. The kit formed by the primer pair further comprises detecting liquid and a micro-fluidic chip; as an independent RT-PCR secondary amplification step of a detecting liquid system is omitted, detecting time is shortened; as the denaturation process and the renaturation process of nucleic acid do not exist, the polluted chance of RNA enzymes and the polluted chance of amplification nucleic acid are reduced, and the sensibility and the safety of detection are improved. By means of the constant-temperature sealed environment provided by a micro-fluidic chip system, rapid and constant-temperature amplification and automation result distinguishing of a nucleic acid extracting template are finally achieved, the requirement for test hardware is reduced, the use level of a reaction reagent is reduced, detection cost is reduced, and the result can be directly determined through color changes.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
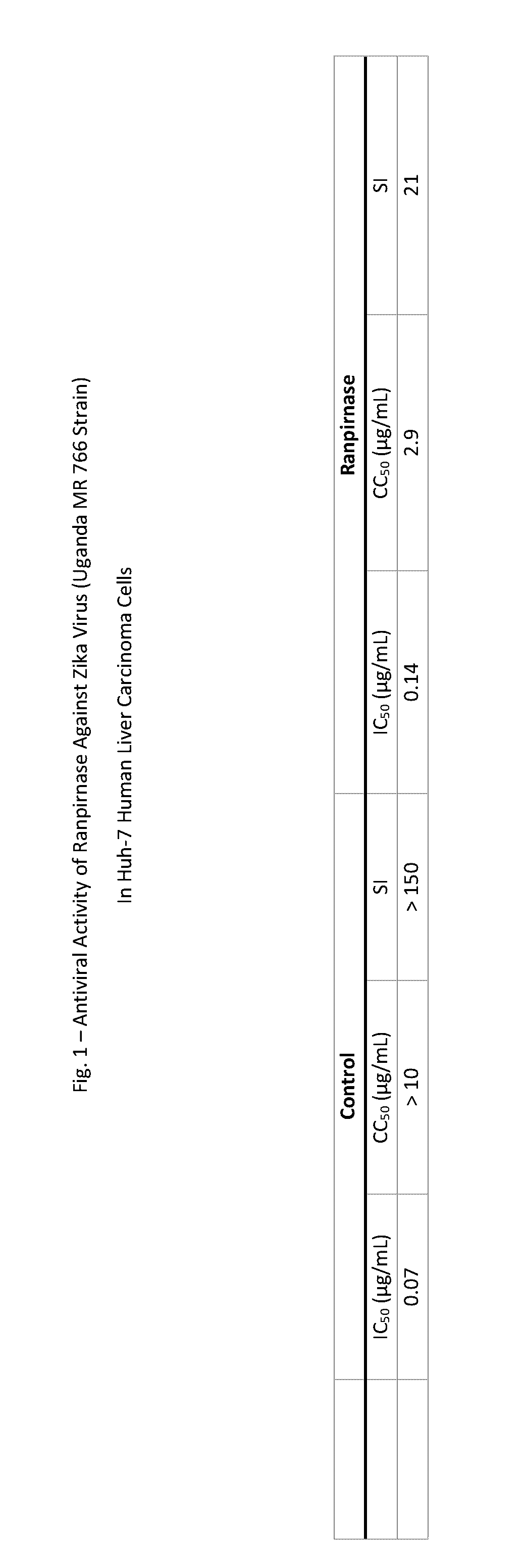
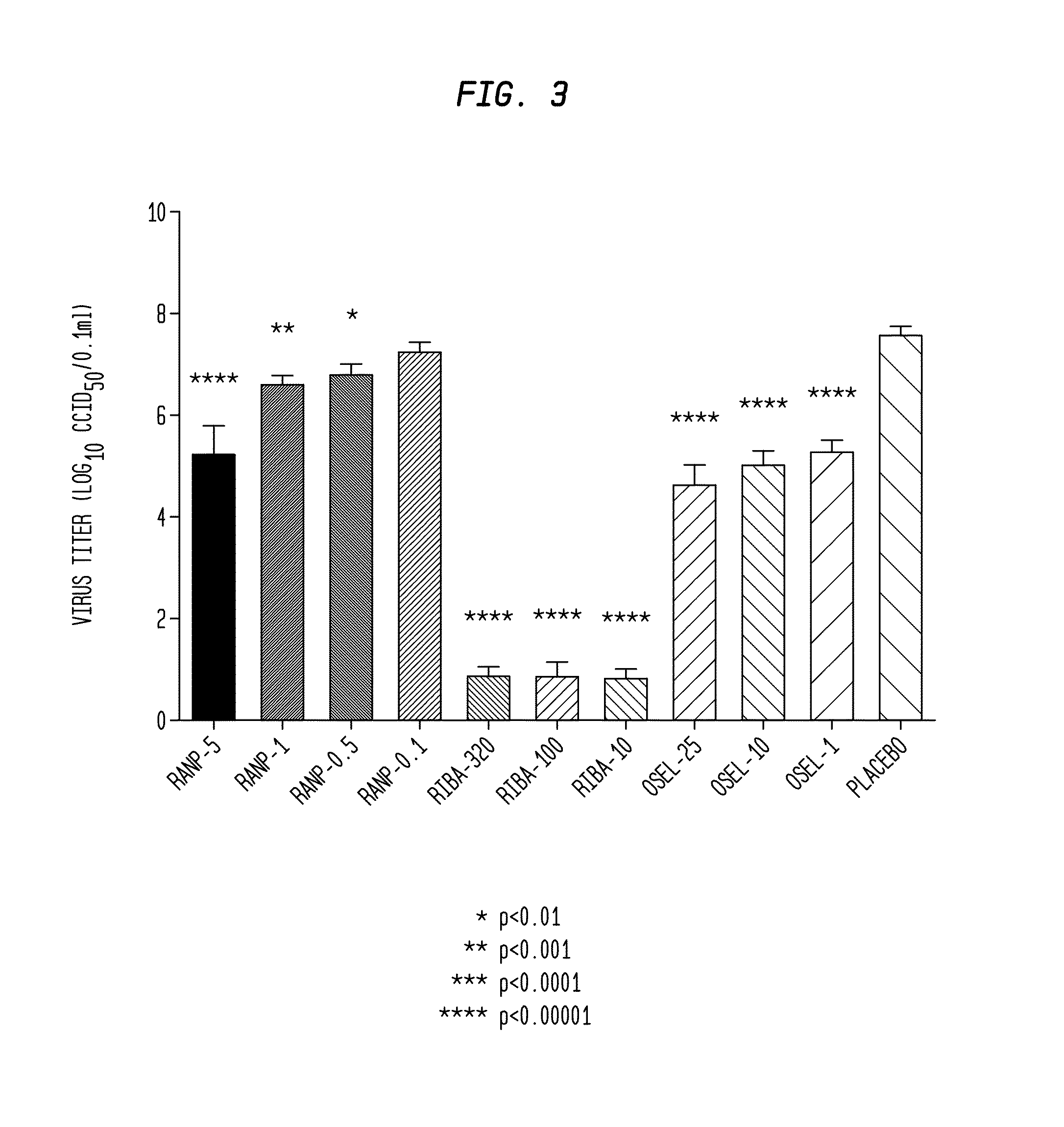
Methods of treating zika virus, mers-cov, chikungunya, venezuelan equine encephalitus, and rhinovirus in mammalian patients
InactiveUS20170157219A1Inhibition formationHydrolasesPeptide/protein ingredientsZika virusMiddle East respiratory syndrome coronavirus
Viral infections in mammals can be treated and prophylactically prevented by systemic administration of ranpirnase and three other ribonucleases that are highly homologous with it and that have activities that are highly similar to it. Experimental results against Zika virus, Middle East Respiratory Syndrome Coronavirus (“MERS-CoV”), Chikungunya virus, Venezuelan equine encephalitis, and rhinovirus-14 are disclosed.
Owner:TAMIR BIOTECH
Multiplex-fluorescence PCR (Polymerase Chain Reaction) detection kit and application thereof
InactiveCN103305633AGuaranteed heightGuaranteed FeaturesMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention relates to the technical field of biological detection, and in particular relates to a primer, a probe and a kit used for detecting dengue fever, chikungunya and yellow fever virus through a single tube by the multiplex-fluorescence PCR (Polymerase Chain Reaction). The primer and probe used for detecting the dengue fever, chikungunya and yellow fever virus through the multiplex-fluorescence PCR have nucleotide sequences as shown in SEQ ID NO: 1-9; the multiplex PCR detection kit for detecting the dengue fever, chikungunya and yellow fever virus comprises multiplex-fluorescence PCR detection mixed liquid which contains the primer and probe mentioned above. The kit, primer and probe can quickly detect the dengue fever virus, chikungunya virus and yellow fever virus, have the advantages of being simple and convenient to operate, high in sensitivity and outstanding in specificity, and can timely find out and confirm a suspect case so as to improve the monitoring level on such diseases.
Owner:浙江国际旅行卫生保健中心 +1
Chikungunya virus isothermal amplification detection kit and primer thereof
InactiveCN102399903ASimple designOptimizing Primer SpacingMicrobiological testing/measurementAgainst vector-borne diseasesFluorescenceChikungunya fever
The invention provides a chikungunya virus isothermal amplification detection kit and a primer thereof. The kit comprises the primer, Bst DNA polymerase, a reaction liquid and SYBR Green I fluorescent dye, wherein the reaction liquid is 10mM deoxyribonucleoside triphosphate, 10*reaction buffer liquid and 150mM MaSO4; the sequences of the primer are sequences represented by SEQ ID NO:1-4 or sequences represented by SEQ ID NO:5-8 or sequences represented by SEQ ID NO:9-12 or sequences represented by SEQ ID NO:13-16. The kit provided by the invention has the advantages of short detection time, strong specificity, high sensitivity, high detection accuracy and programmed reaction, is easy and convenient to qualify and is widely applied to conventional detection and epidemiological survey in clinic and ports.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
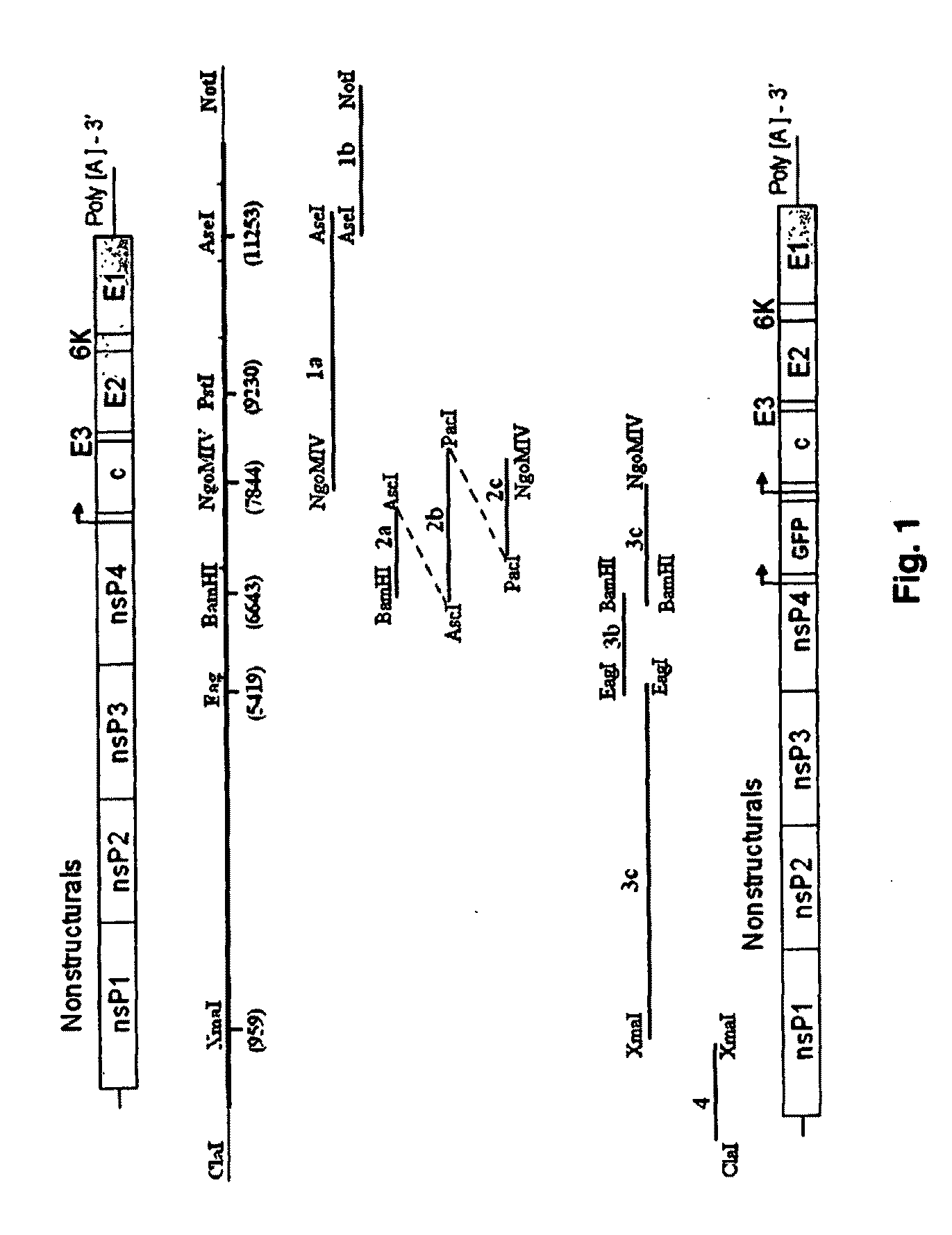
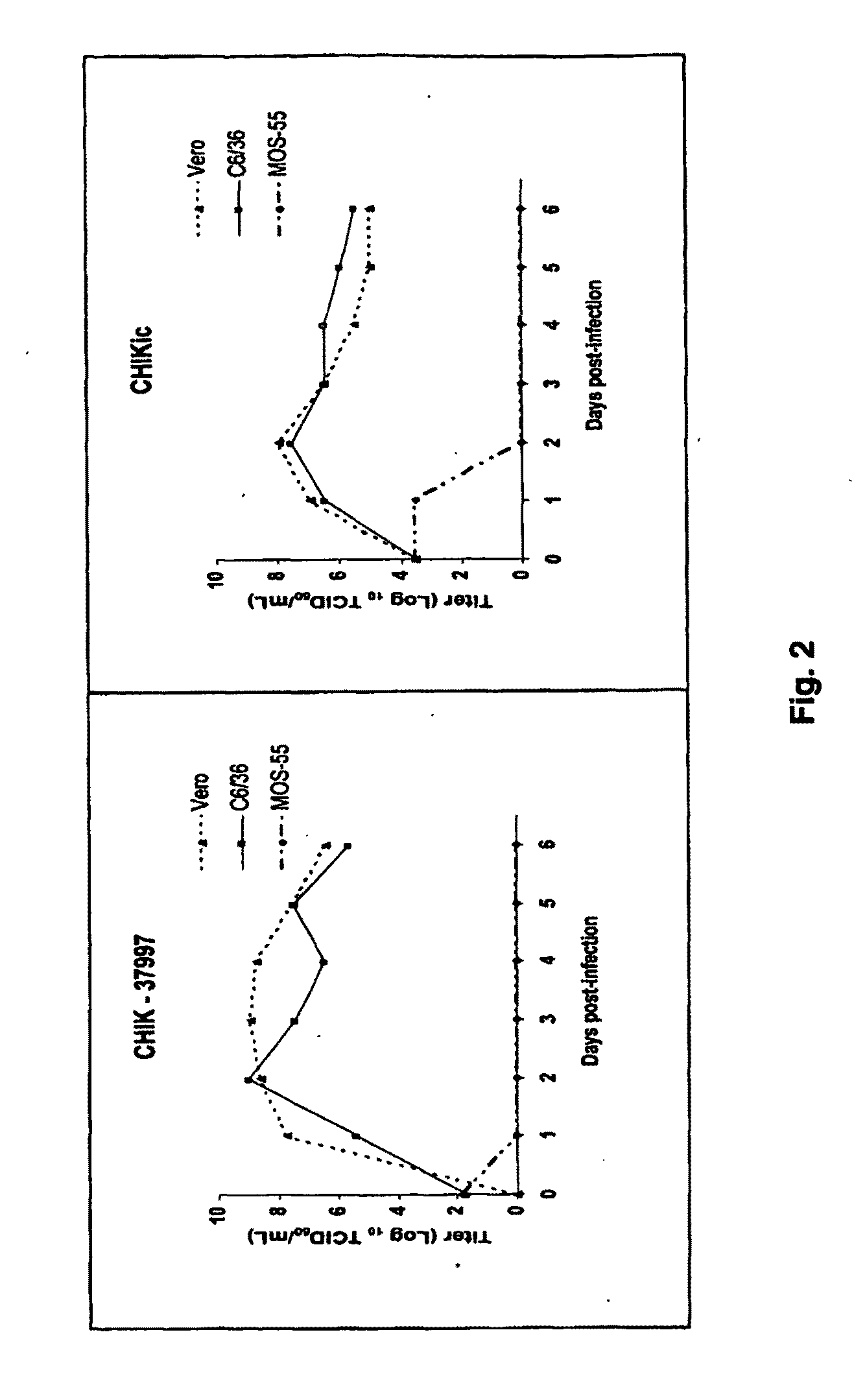
Chikungunya virus infectious clones and uses therefor
InactiveUS20100233209A1High responseHigh infection rateSsRNA viruses positive-senseSugar derivativesDelivery vehicleFull length cdna
The present invention developed and characterized in vitro and in vivo three full-length cDNA clones based on the alphavirus chikungunya, two sets of infectious clones based on CHIKV and replicons based on the principle used to generate the infection clones. Described herein is the method to generate such infective clones and replicons, their composition and their use as molecular tool, a delivery vehicle and vaccine.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Kit for joint detection on dengue fever virus and chikungunya virus on basis of fluorescent PCR method
InactiveCN106755565AHighly conservativeImprove featuresMicrobiological testing/measurementAgainst vector-borne diseasesForward primerChikungunya fever
The invention relates to the field of PCR detection kits, in particular to a kit for joint detection on a dengue fever virus and a chikungunya virus on the basis of a fluorescent PCR method. The kit for joint detection on the dengue fever virus and the chikungunya virus comprises a specific primer for the dengue fever virus, a dengue fever virus probe, a specific primer for the chikungunya virus and a chikungunya virus probe, wherein the specific primer for the dengue fever virus comprises a forward primer and a reverse primer; the specific primer for the chikungunya virus comprises a forward primer and a reverse primer. The kit provided by the invention specifically relates to two pairs of specific PCR primers and probers for respectively specifically amplifying the dengue fever virus and the chikungunya virus, the two pairs of the specific PCR primers can amplify the viruses simultaneously in a same PCR reaction system, multi-PCR detection is achieved, and not only is operation simple and convenient, but also the detection time is greatly shortened.
Owner:广东省疾病预防控制中心 +1
Methods of treating viral infections, particularly rabies, mers-cov, influenza, ebola, chikungunya, venezuelan equine encephalitus, canine parvovirus, adenovirus, respiratory syncytial virus, rhinovirus, and poxvirus in mammalian patients
Viral infections in mammals can be treated and prophylactically prevented by systemic administration of ranpirnase and three other ribonucleases that are highly homologous with it and that have activities that are highly similar to it. Experimental results against rabies, Middle East Respiratory Syndrome Coronavirus (“MERS-CoV”), influenza, Ebola virus, Chikungunya virus, Venezuelan equine encephalitis, canine parvovirus, adenovirus-2, respiratory syncytial virus, rhinovirus-14, and vaccinia are disclosed.
Owner:ORGENESIS INC
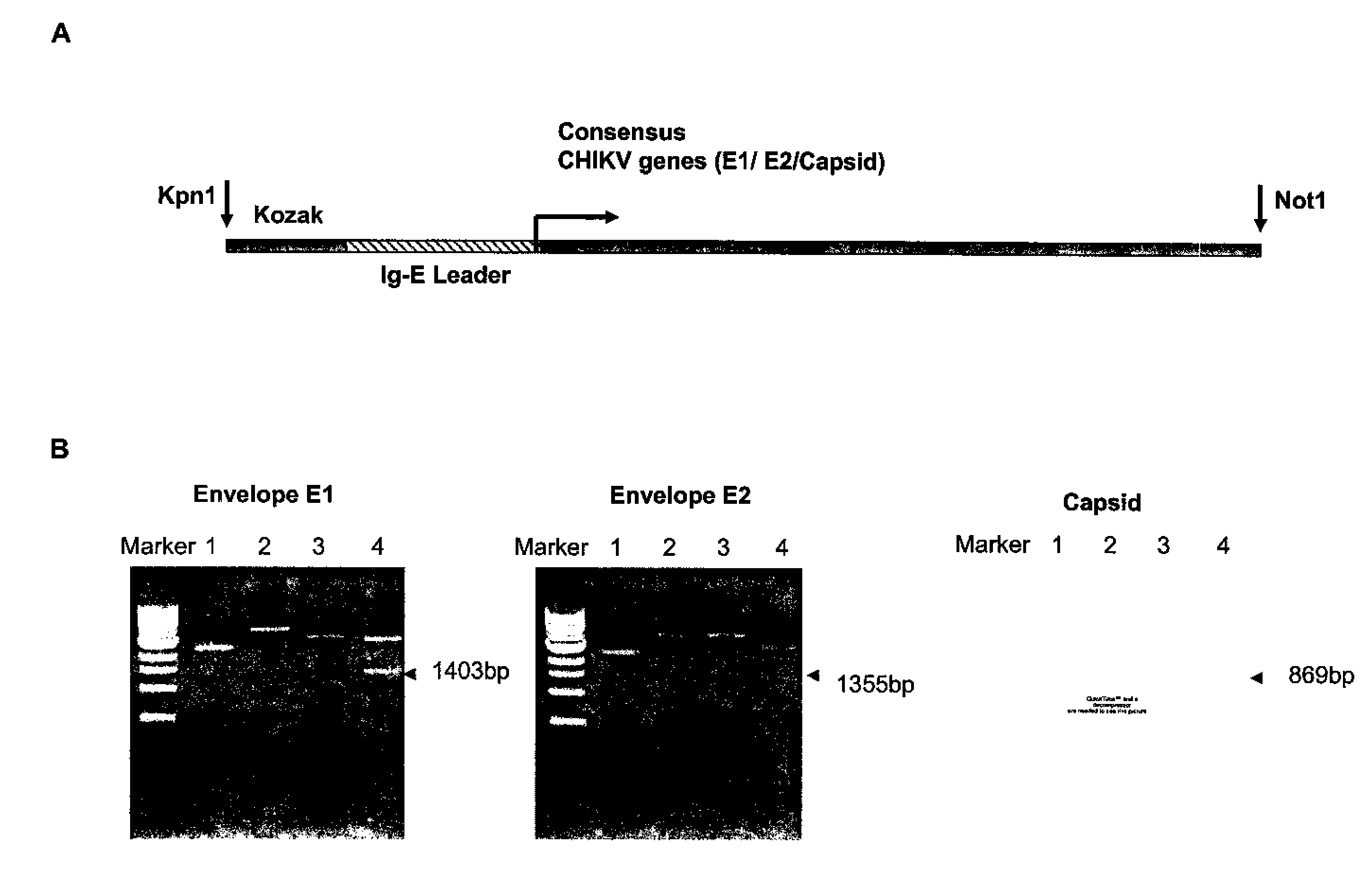
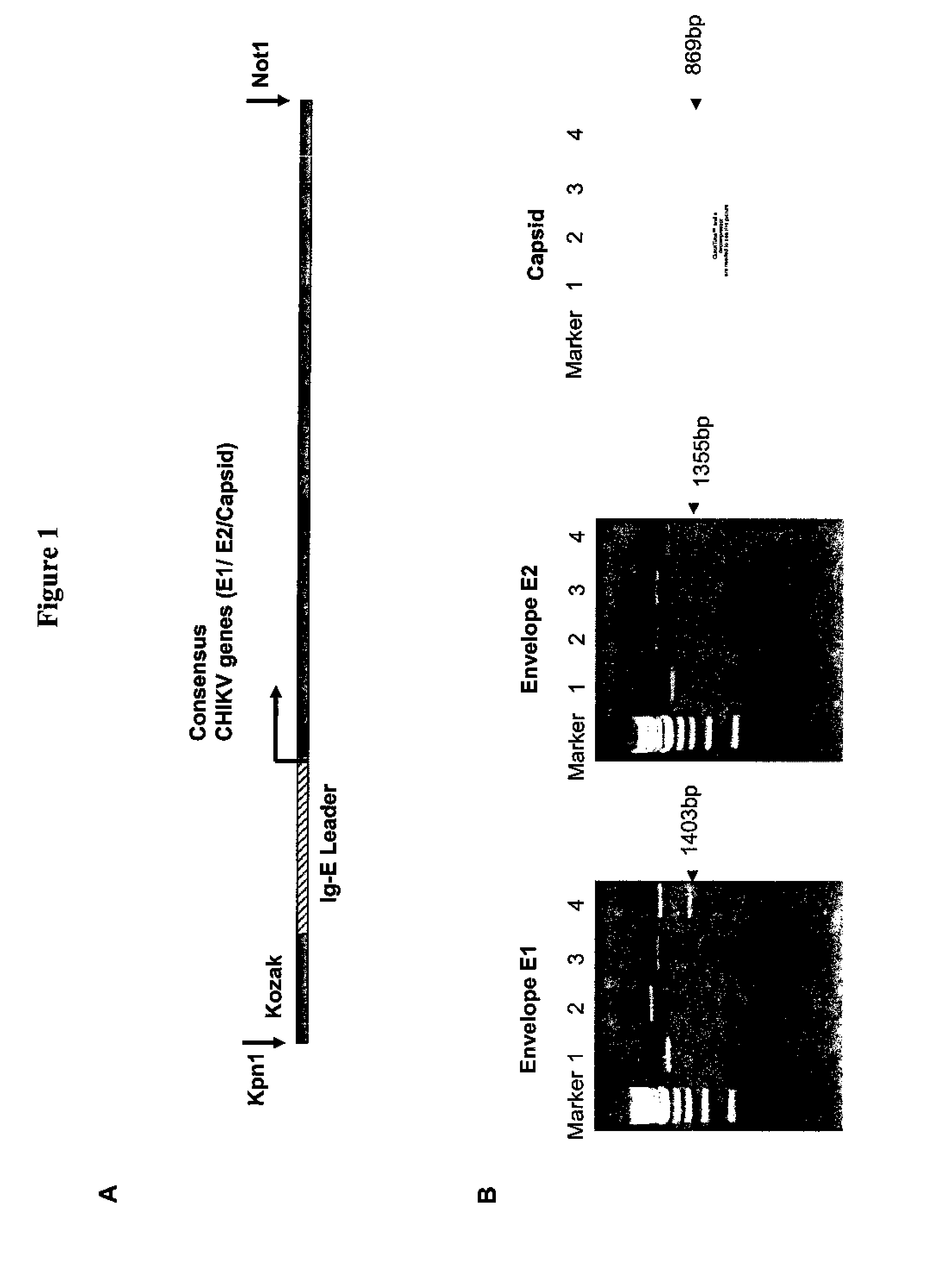
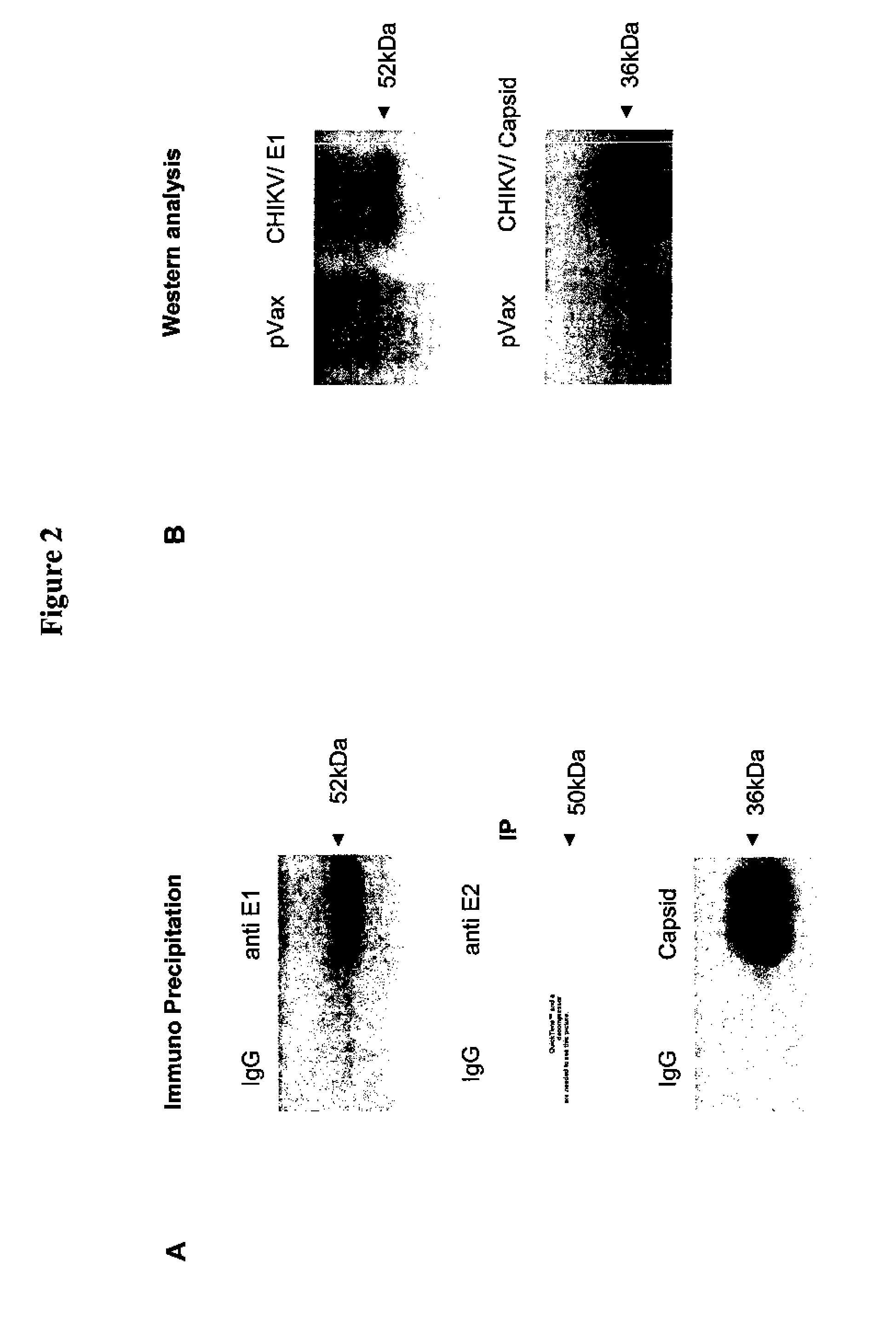
Consensus sequences of chikungunya viral proteins, nucleic acid molecules encoding the same, and compositions and methods for using the same
Consensus CHIKV E1 protein, consensus CHIKV E2 protein, consensus CHIKV capsid protein, or fragments and homologues thereof, and nucleic acid molecules that encode the same are disclosed. A consensus CHIKV Env protein which includes CHIKV E1 consensus protein, CHIKV E2 consensus protein, CHIKV E3 consensus protein, or fragments and homologues thereof and nucleic acid molecules that encode the same are also disclosed. Compositions and recombinant vaccines comprising CHIKV consensus proteins, and methods of using them are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
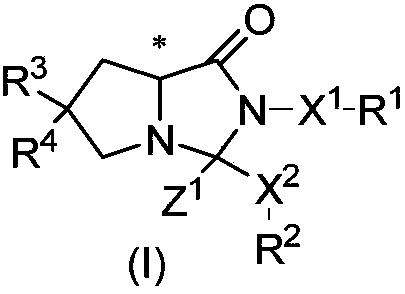
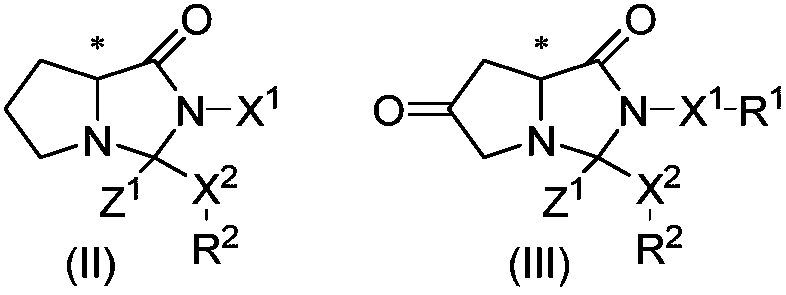
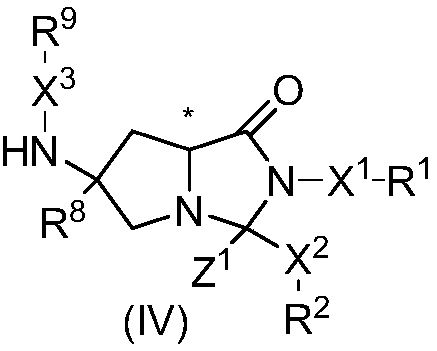
Cyclocompound of tetrahydropyrrole and dihydroimidazolone as well as preparation and pharmaceutical application thereof
The invention discloses a cyclocompound of tetrahydropyrrole and dihydroimidazolone as well as preparation and pharmaceutical application thereof. The cyclocompound is a compound shown in a formula (I), an isomer or pharmaceutically acceptable salt thereof. The compound, isomer or pharmaceutically acceptable salt thereof can be applied to preparation of medicines used for preventing or treating related diseases (such as dengue fever, dengue hemorrhagic fever, dengue shock syndrome, Zika, Chikungunya, Japanese encephalitis, yellow fever, hepatitis C and West Nile disease) caused by a dengue fever virus and related viruses. (The formula (I) is described in the specification).
Owner:NANJING TECH UNIV
Antiviral traditional Chinese medicine composition and preparation method and application thereof
ActiveCN107184885AImprove securityGood antiviral effectAntiviralsPlant ingredientsHigh resistanceInfluenza h1n1
The invention belongs to the field of drug research and development, and relates to an antiviral traditional Chinese medicine composition and a preparation method and application thereof. The composition comprises Mongolian dandelion herb, root of sessile stemona and Indian iphigenia bulb and further optionally comprises root of lobed kudzuvine, rhizome of largehead atractylodes and root of blackend swallowwort, and can be prepared into appropriate pharmaceutically acceptable drug forms as needed. The composition has high resistance activity to strains of various acute infectious diseases and has good drug safety; as in-vitro(cell) pharmacodynamic test and in-vivo experiment result proved, the composition has effective broad-spectrum antiviral effect to viruses of influenza A H1N1, H7N7 and H9N2, Zika virus, Dengue fever type I and type II viruses, Chikungunya virus and the like; in addition, the composition can be developed into an oral broad-spectrum plant compound resisting various infectious viruses, which will be the major innovative breakthrough of clinic treatment in the related disease field.
Owner:ジアホンチャン
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com