Anti-SARS-CoV-2 single-chain antibody, and preparation method and application thereof
A single-chain antibody, sars-cov-2 technology, applied in the field of biomedicine, can solve the problems of side effects, difficulty in large-scale application, high production cost of human neutralizing antibodies, etc., achieve high virus neutralization activity, not easy to dissociate, The effect of low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
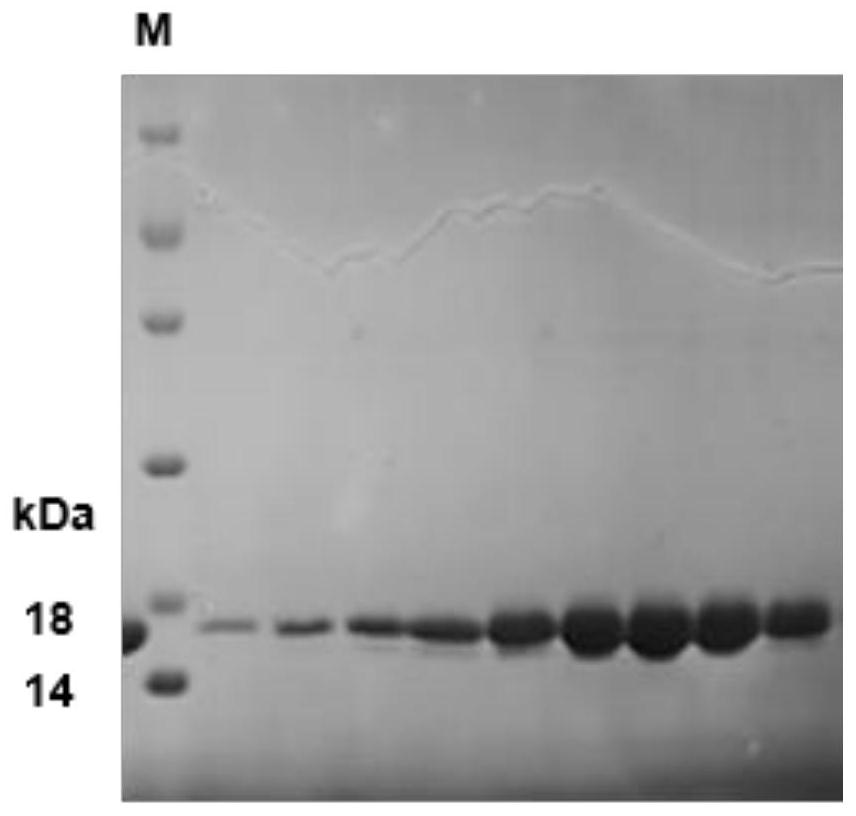
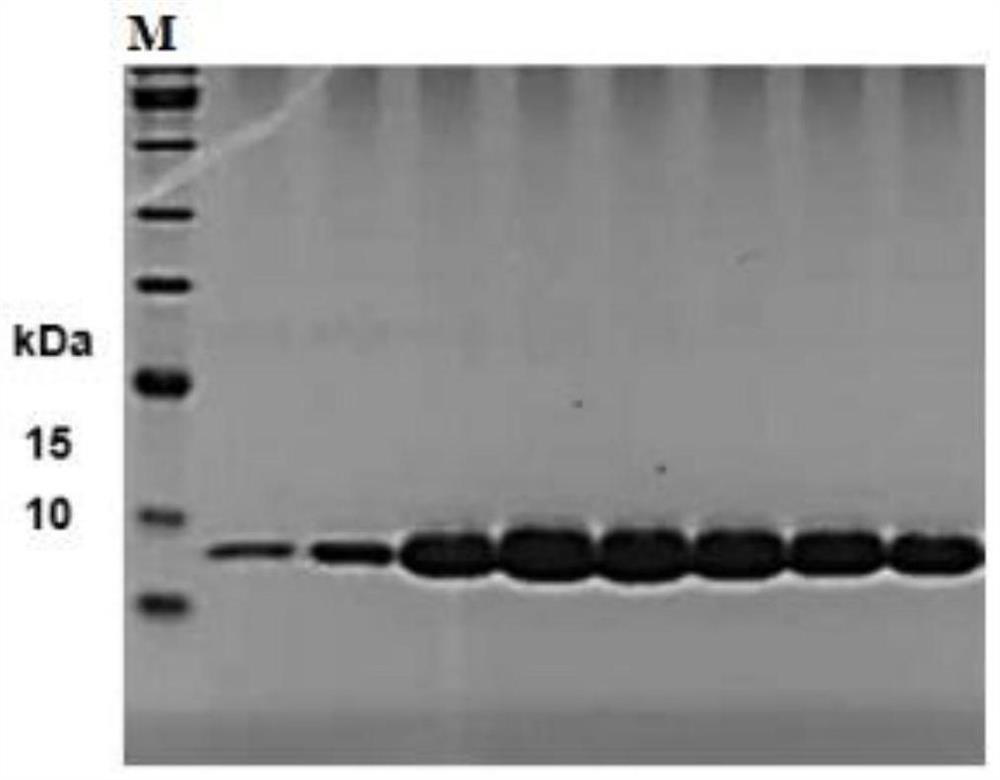
[0078] This embodiment constructs a single-chain antibody phage library, and the construction method of the single-chain antibody phage library includes the following steps:
[0079] (1) Dissolve 100 μg of SARS-CoV-2S1 protein (purchased from Beijing Yiqiao Shenzhou) in 250 μL of PBS, emulsify with an equal volume of aluminum adjuvant, and inject subcutaneously and intramuscularly at multiple points to immunize Bamboo shark. times, with an interval of 2 weeks each time;
[0080] (2) From the second immunization, 1 mL of blood was collected from the tail vein one week after each immunization, 0.5 mL of which was treated with anticoagulation to separate lymphocytes, lysed with trizol, and stored at -80°C for later use; another 0.5 mL was taken and left at room temperature for 1 hour , centrifuge serum to detect serum titer, tail vein blood collection after the last immunization to detect serum pseudovirus neutralization titer;
[0081] (3) Take the lymphocyte lysate after the 3...
Embodiment 2
[0090] In this embodiment, the screening and expression of SARS-CoV-2 single-chain antibody comprises the following steps:
[0091] (1) Take 100 μL of the single-chain antibody phage library prepared in Example 1, inoculate it in 50 mL of 2YT medium containing ampicillin antibiotics and 1% glucose, cultivate to the logarithmic phase at 37° C., add 20 times the number of helper phage M13KO7, After mixing, let stand at 37°C for 20 minutes, shake and culture for 30 minutes, centrifuge, discard the medium, add 50mL 2YT ampicillin kanamycin medium, shake and culture at 30°C overnight, centrifuge the next day, and collect the supernatant , add 1 / 4 volume of PEG / NaCl, precipitate the recombinant phage at 4°C for 2 hours, collect the phage precipitate by centrifugation, dissolve it with 5mL PBS, repeat the precipitation once, dissolve the phage with PBS, add glycerol with a final concentration of 15%, and store in aliquots Store at -80°C for later use, and at the same time take 10 μL ...
Embodiment 3
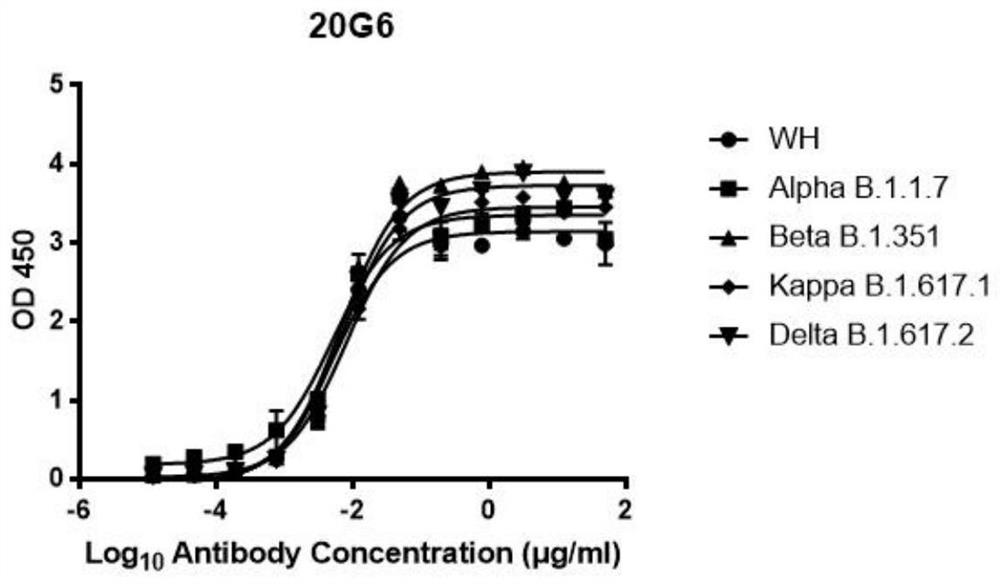
[0100] This embodiment detects the binding ability of the SARS-CoV-2 single-chain antibody prepared in Example 2, including the following steps:
[0101] (1) The RBD (receptor binding site domain in the spike protein, which is the 332nd to 527th amino acid) proteins (WH-Hu-1 strain, British strain B.1.1.7 (Alpha , including N501Y mutation site), South African strain B.1.351 (Bbeta, including three site mutations K417N, E484K, N501Y) and Indian strain B.1.617 (Kappa, including L452R, E484Q mutation sites), B.1.617. 2 (Delta, including L452R, T478K mutation sites)) diluted in CBS buffer, coated with ELISA plate, 50ng / 100μL / well, incubated at 4°C for 12 hours, discarded the antigen, added blocking solution (5% skimmed milk Dissolve in PBST), 200 μL / well, block at 37°C for 2 hours, wash the plate, 0.05% PBST, 200 μL / well, wash 4 times, 2 minutes each time, shake off the liquid, and pat dry;
[0102] (2) Dilute the 20G6 and 17F6 antibodies in PBST, starting from 100 μg / mL, make se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com