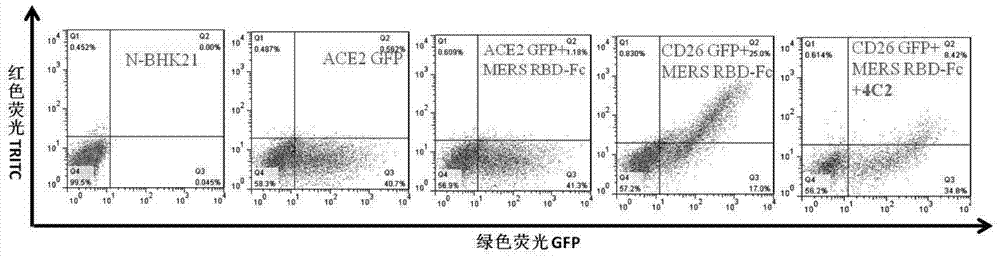
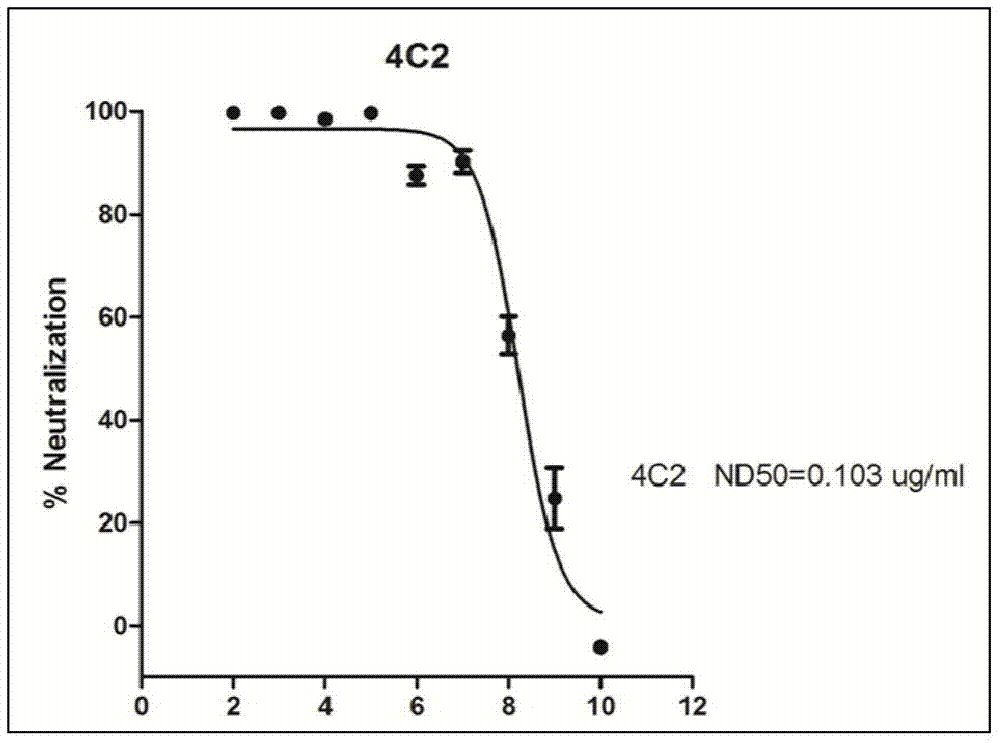
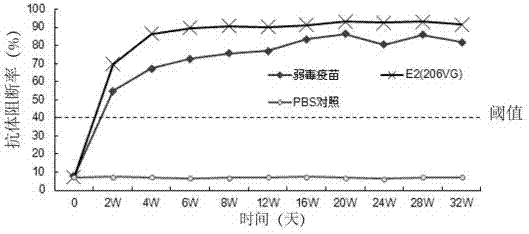
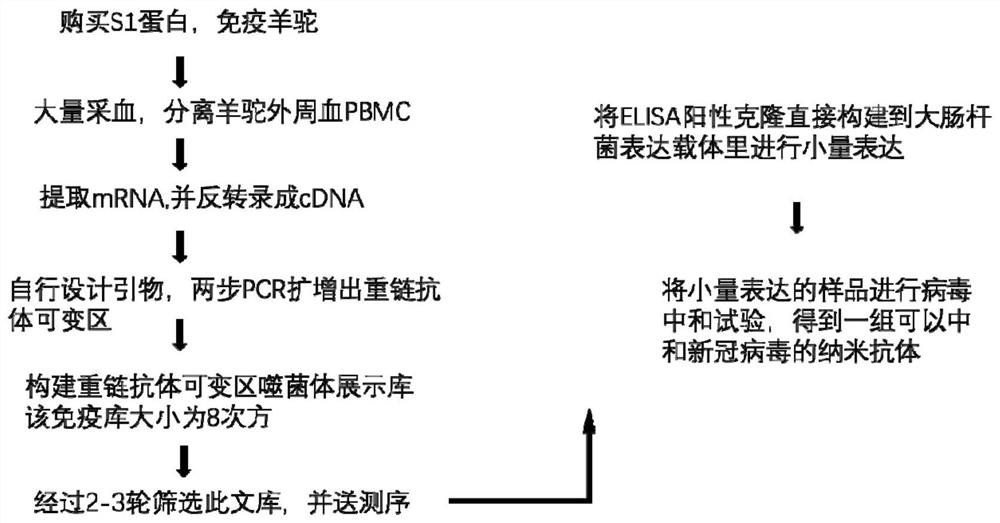
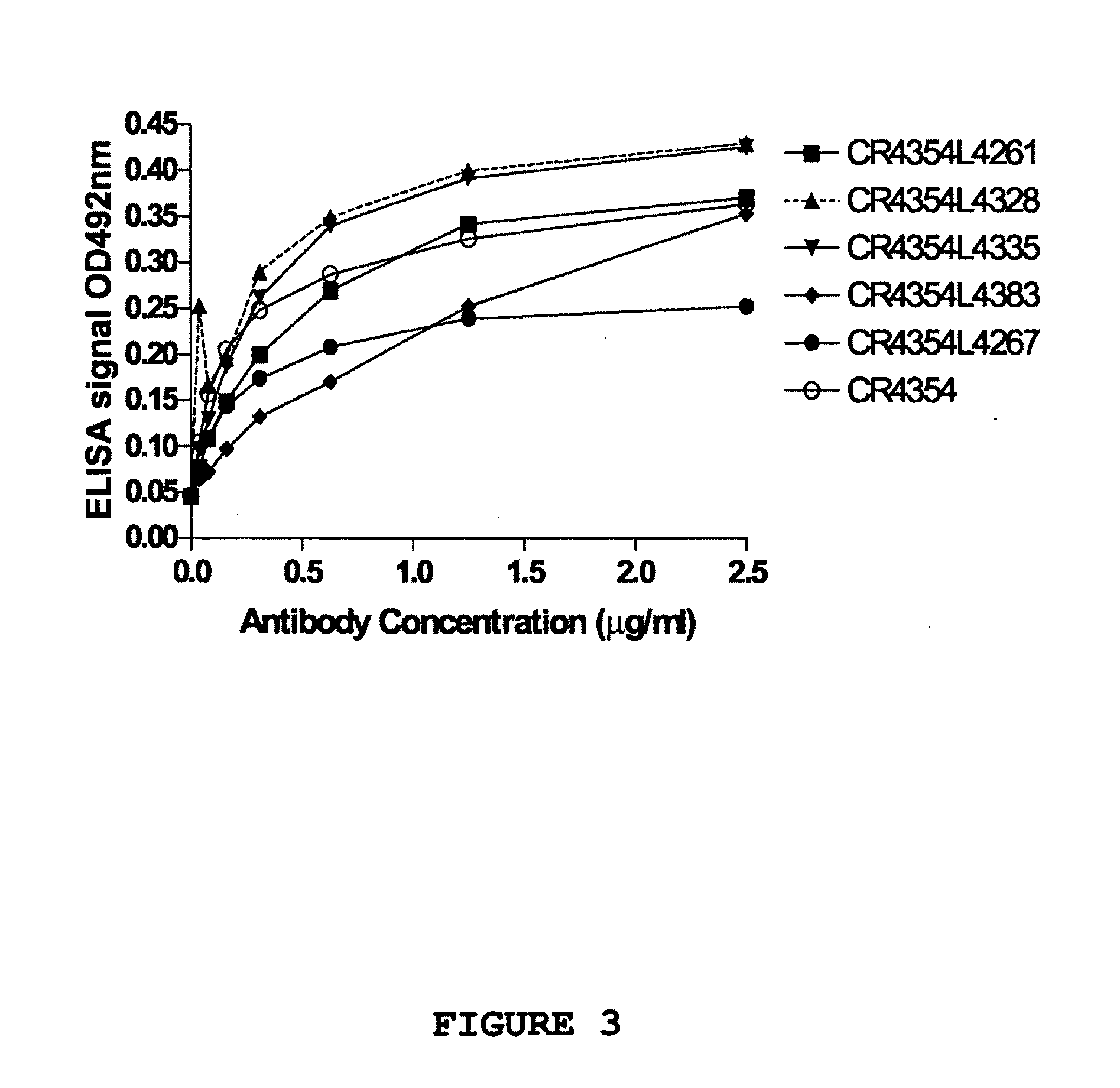
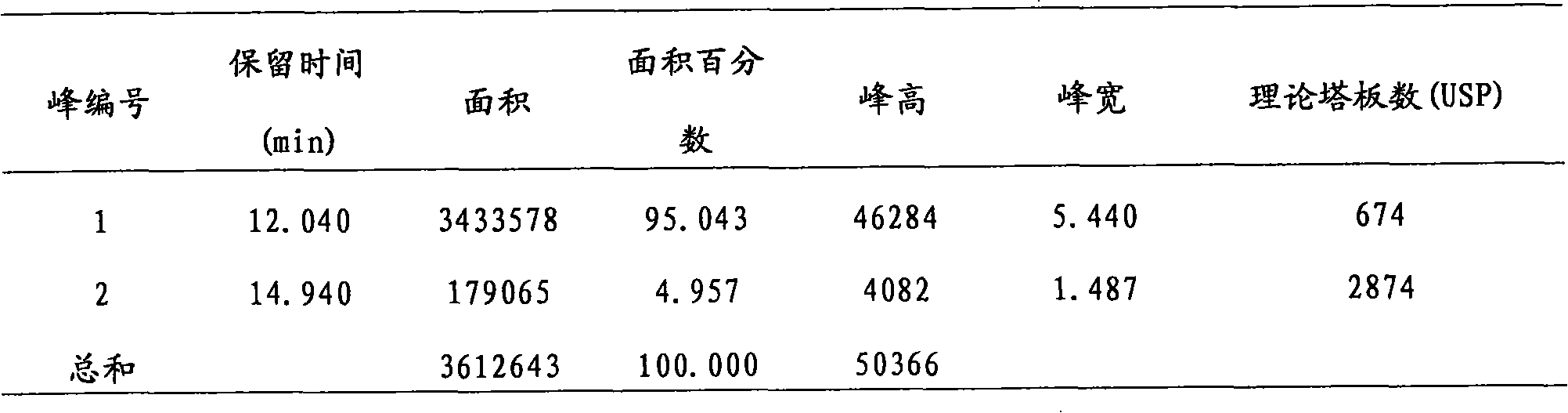
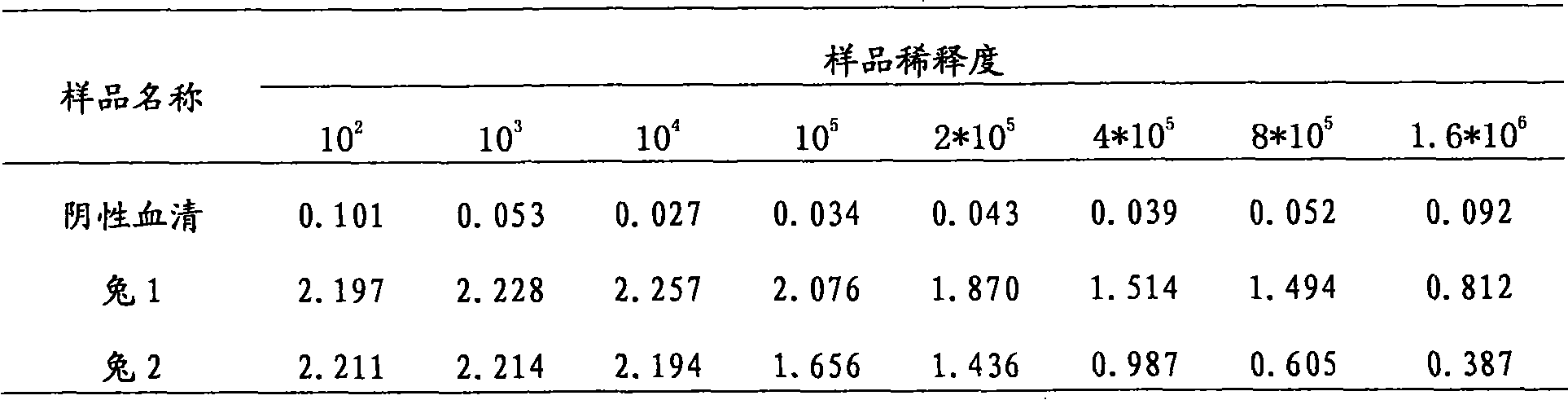
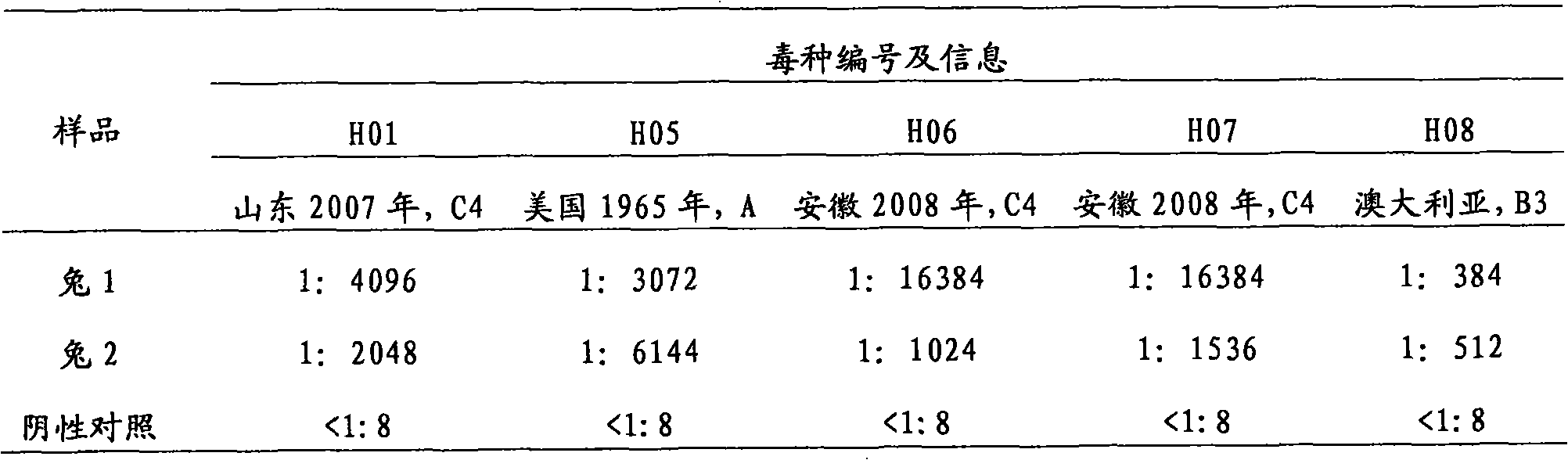
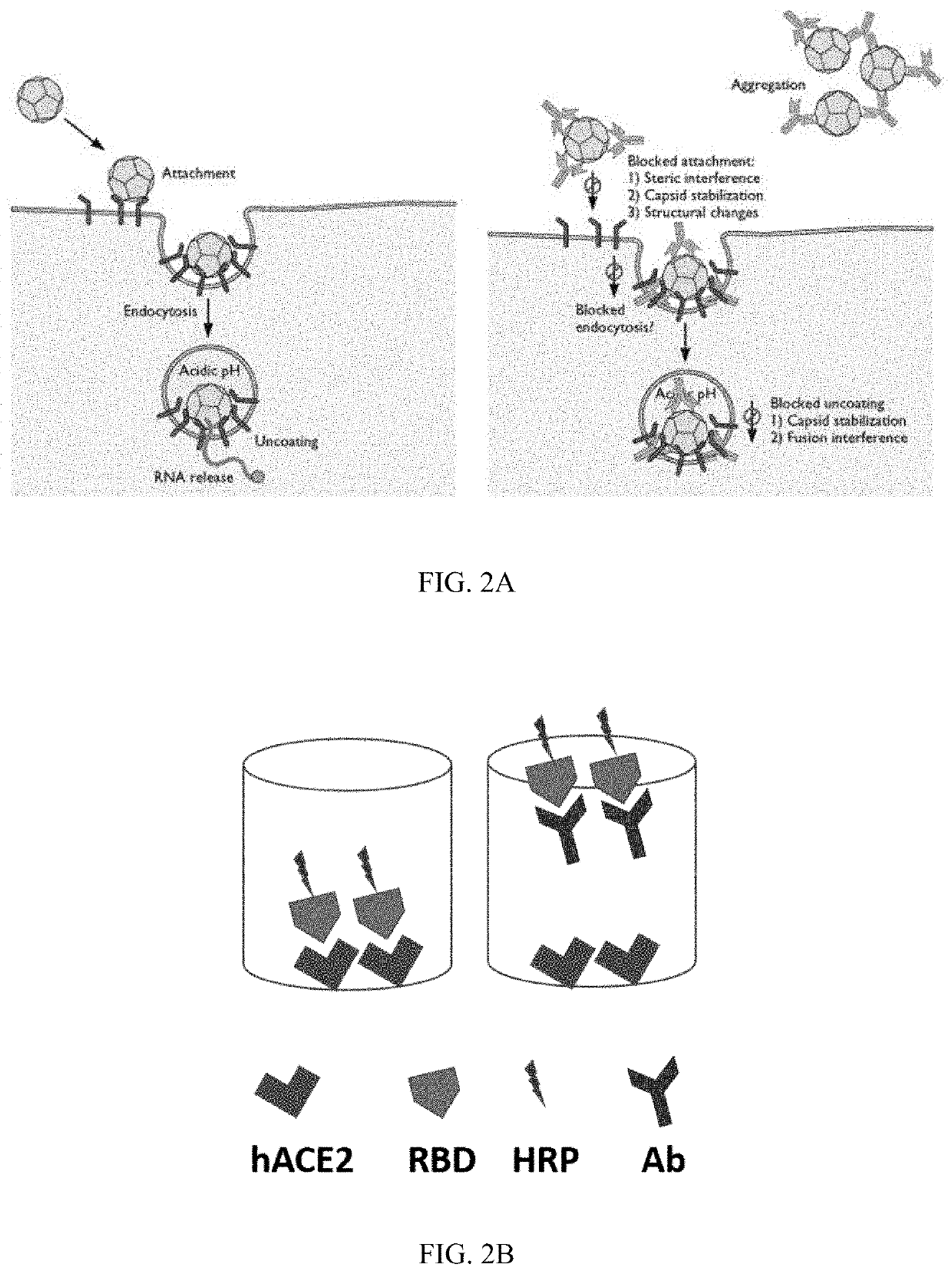
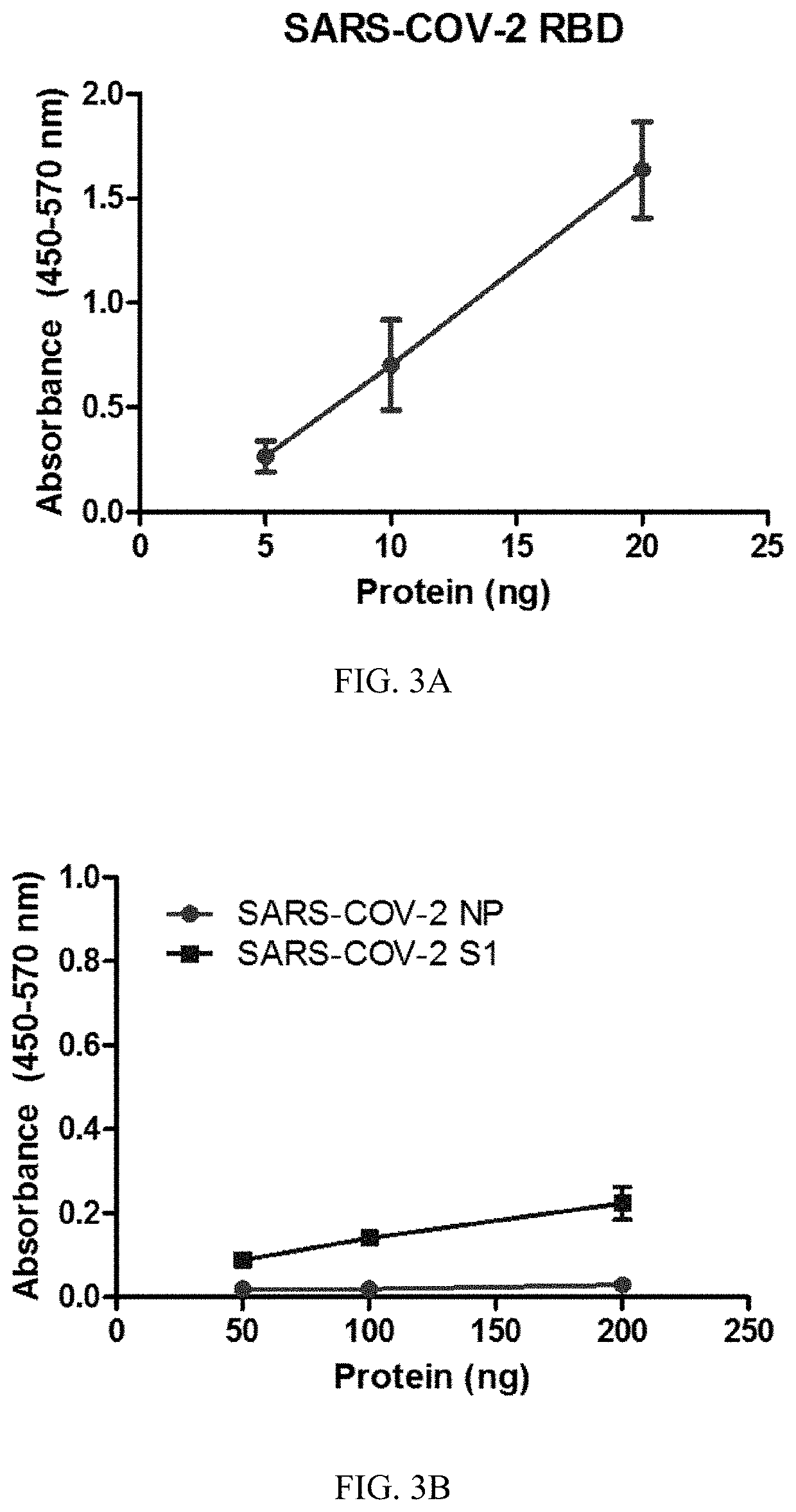
Patents
Literature
130 results about "Virus Neutralization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Neutralization of a virus is defined as the loss of infectivity through reaction of the virus with specific antibody.
Neutralizing antibody for resisting novel coronavirus SARS-Cov-2 and application thereof
ActiveCN111592595ABlock bindingGood effectImmunoglobulins against virusesAntiviralsNeutralizing antibodyPhage Display Techniques
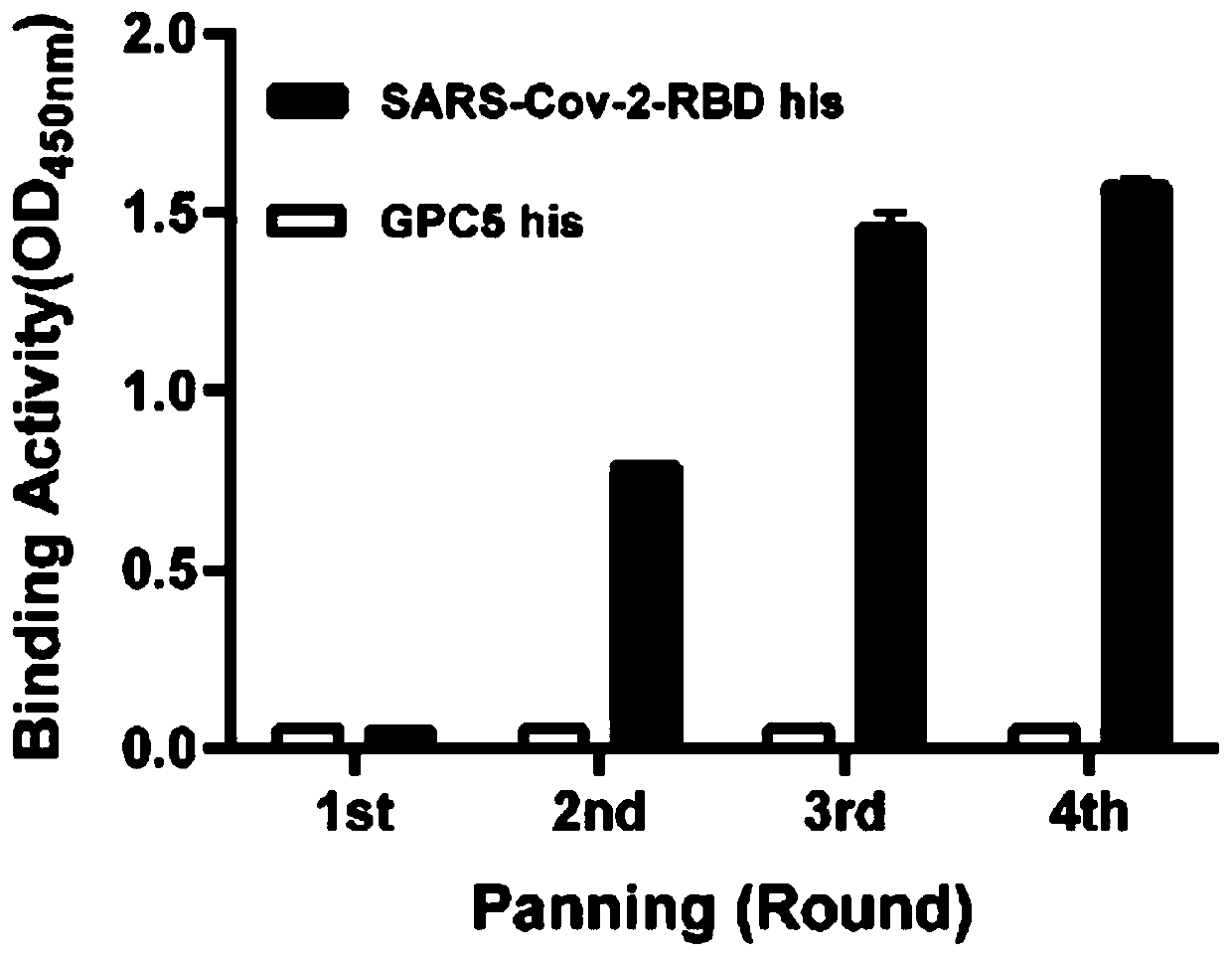
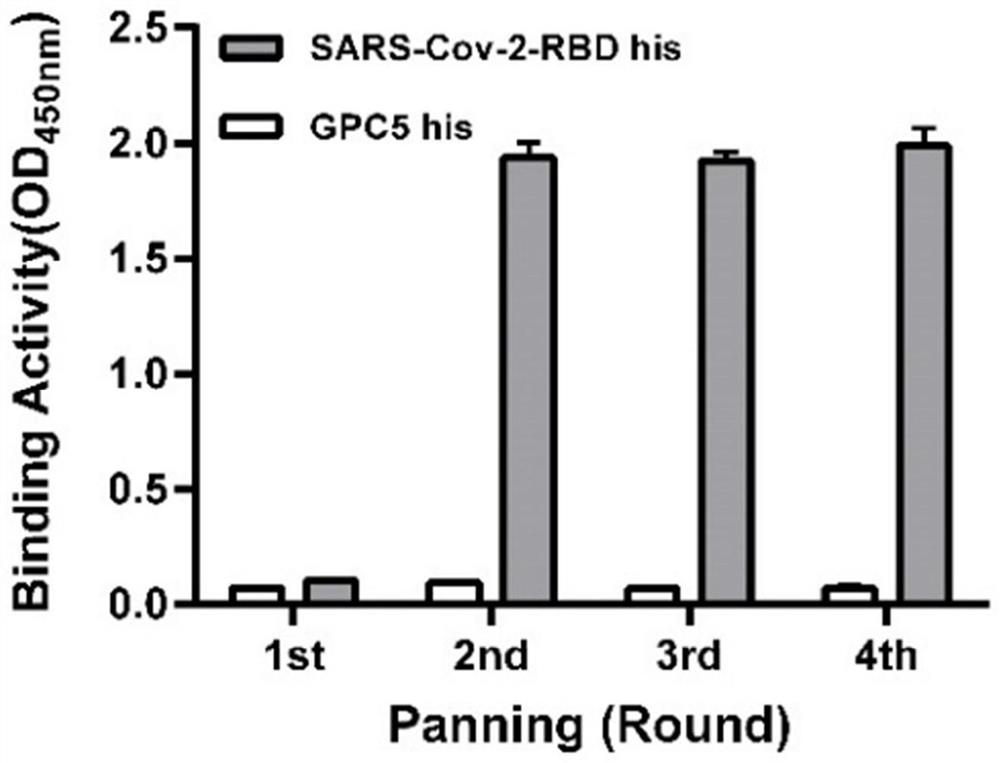
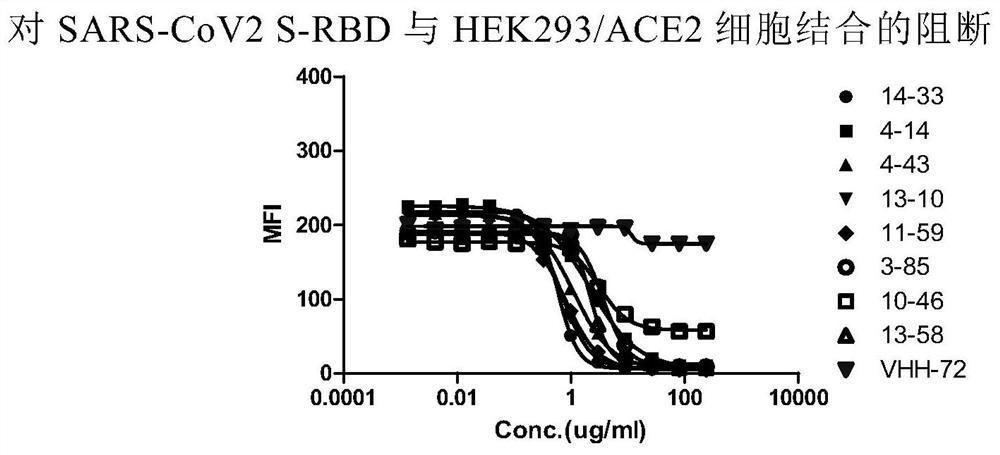
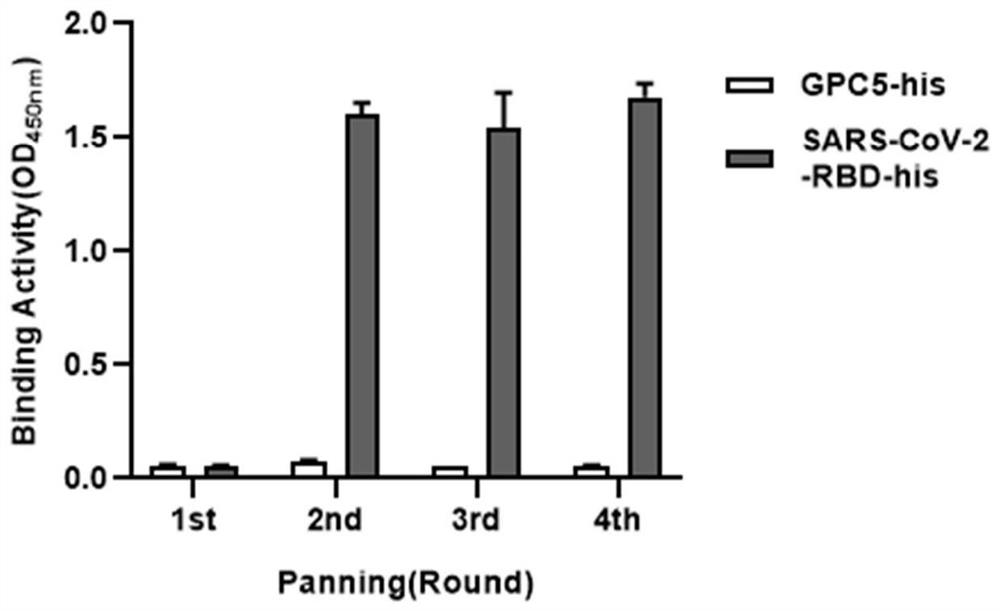
The invention relates to a neutralizing antibody for resisting a novel coronavirus SARS-Cov-2 and an application of the neutralizing antibody. The antibody at least comprises one of a heavy chain CDR1, a heavy chain CDR2, a heavy chain CDR3, a light chain CDR1, a light chain CDR2 and a light chain CDR3. The antibody can be used for preparing a diagnostic reagent or a diagnostic kit, a drug or a pharmaceutical composition for detecting, preventing and treating a COVID-19. According to the neutralizing antibody, differential antibody screening is carried out through a phage display technology ina manner of targeting SARS-Cov-2-RBD and SARS-Cov-1-RBD; the neutralizing antibody for resisting the novel coronavirus SARS-Cov-2 is obtained; binding of the SARS-Cov-2-RBD and ACE2 positive cells can be blocked; and the neutralizing antibody has a remarkable virus neutralizing effect on an SARS-Cov-2 pseudo virus and provides an effective alternative antibody drug for prevention and treatment ofthe COVID-19.
Owner:NANJING MEDICAL UNIV
Middle east and respiratory syndrome coronavirus antibody and preparation method thereof
ActiveCN103864924APrevent intrusionHigh affinityImmunoglobulins against virusesAntibody ingredientsMiddle East respiratory syndromeBaculovirus expression vector system
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
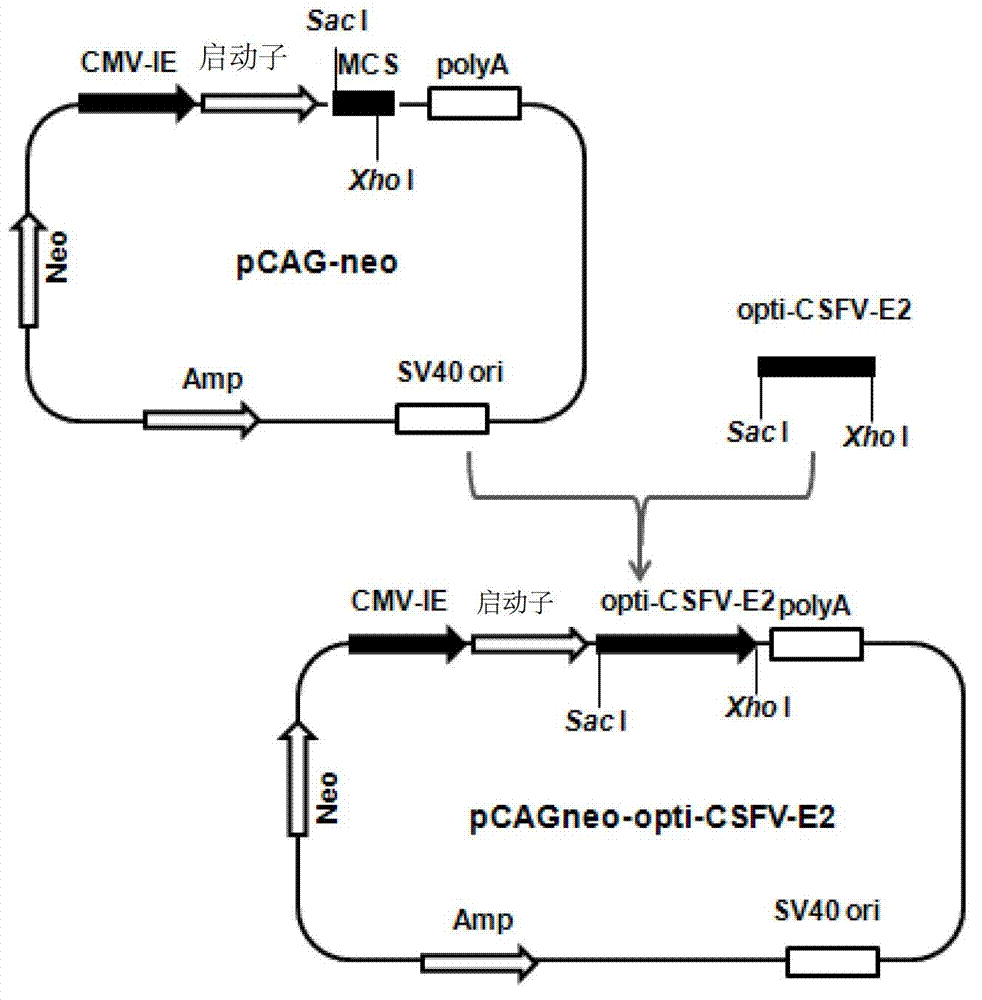
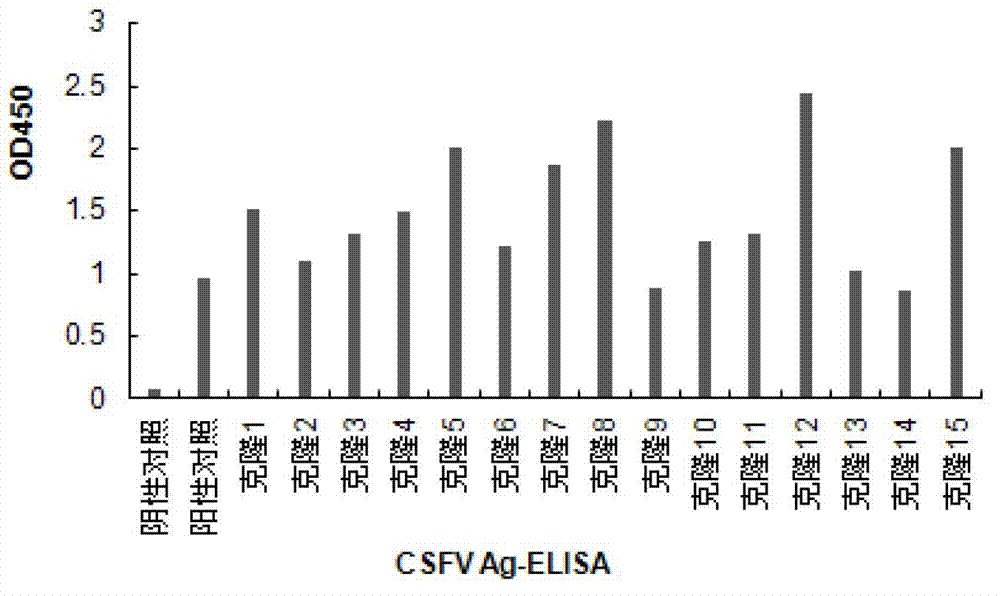
Recombinant cell line for stably expressing classical swine fever virus E2 protein, and applications of the same in preparation of subunit vaccines and diagnosis reagents of classical swine fever
ActiveCN103751774AStable in natureFight infectionMicroorganism based processesAntiviralsMaternal antibodyAntigen
The present invention discloses a strain of a recombinant cell line for stably expressing classical swine fever virus E2 protein, and applications of the recombinant cell line in preparation of subunit vaccines and diagnosis reagents of classical swine fever, wherein specifically the recombinant cell line is BCSFV-E2, is preserved in the China General Microbiological Culture Collection Center, and has the preservation number of CGMCC No.7719. The classical swine fever subunit vaccine prepared by using the recombinant cell line has characteristics of high safety, good immunization effect, easy mass production, less being susceptible to exogenous virus pollution or influence of antibodies, and no influence of the maternal antibody on immunization of swine, and can induce and produce high level classical swine fever virus neutralization antibodies after the swine is immunized. In addition, the present invention further discloses a method for constructing the recombinant mammalian cell line, a method for preparing the classical swine fever subunit vaccine, and applications of the antigen expressed by the recombinant cell line in preparation of classical swine fever prevention vaccines and diagnosis reagents.
Owner:HARBIN WEIKE BIOTECH DEV +1
Novel coronavirus SARS-Cov-2 resistant neutralizing single-domain antibody and application thereof
ActiveCN111647076AGood effectEffective Alternative Antibody DrugsBiological material analysisImmunoglobulins against virusesSingle-domain antibodyPhage Display Techniques
The invention relates to a novel coronavirus SARS-Cov-2 resistant neutralizing single-domain antibody and application thereof. The antibody at least has one of heavy chain CDR1, heavy chain CDR2 and heavy chain CDR3, and can be used for preparing diagnostic reagent or diagnostic kit, an antibody medicine or medicinal composition against COVID-19. The novel coronavirus SARS-Cov-2 resistant neutralizing single-domain antibody is obtained through a phage display technology, can stop combination of the SARS-Cov-2-RBD and ACE2 positive cells, has a remarkable virus neutralizing effect on SARS-Cov-2pseudovirion, and provides an efficient backup antibody medicine for COVID-19 prevention and treatment.
Owner:NANJING MEDICAL UNIV
Nano antibody for neutralizing toxicity of novel coronavirus as well as preparation method and application of nano antibody
ActiveCN112010967AReduce manufacturing costEnhanced inhibitory effectBiological material analysisImmunoglobulins against virusesComplementarity determining regionVirus Neutralization
The invention discloses a nano antibody for neutralizing toxicity of a novel coronavirus as well as a preparation method and application of the nano antibody. The nano antibody comprises a framework region FR and a complementarity determining region CDR, wherein the complementary determining region CDR comprises a CDR1, a CDR2 and a CDR3; the amino acid sequence of the CDR1 is selected from at least one of amino acid sequences shown as SEQ ID NO.1 and SEQ ID NO.2; the amino acid sequence of the CDR2 is selected from at least one of amino acid sequences shown as SEQ ID NO.3, SEQ ID NO.4 and SEQID NO.5; and the amino acid sequence of the CDR3 is selected from at least one of amino acid sequences shown in any one of SEQ ID NO.6 to SEQ ID NO.9. The nano antibody for neutralizing toxicity of novel coronavirus has the advantages of small molecular weight, high affinity with SARS-CoV-2 virus, low production cost and the like.
Owner:SYSVAX INC
Porcine reproductive and respiratory syndrome bivalence recombinant adenovirus vaccine and preparation method thereof
InactiveCN101380468AImmediately exert cellular immune functionNot pathogenicViral antigen ingredientsAntiviralsEukaryotic plasmidsAttenuated vaccine
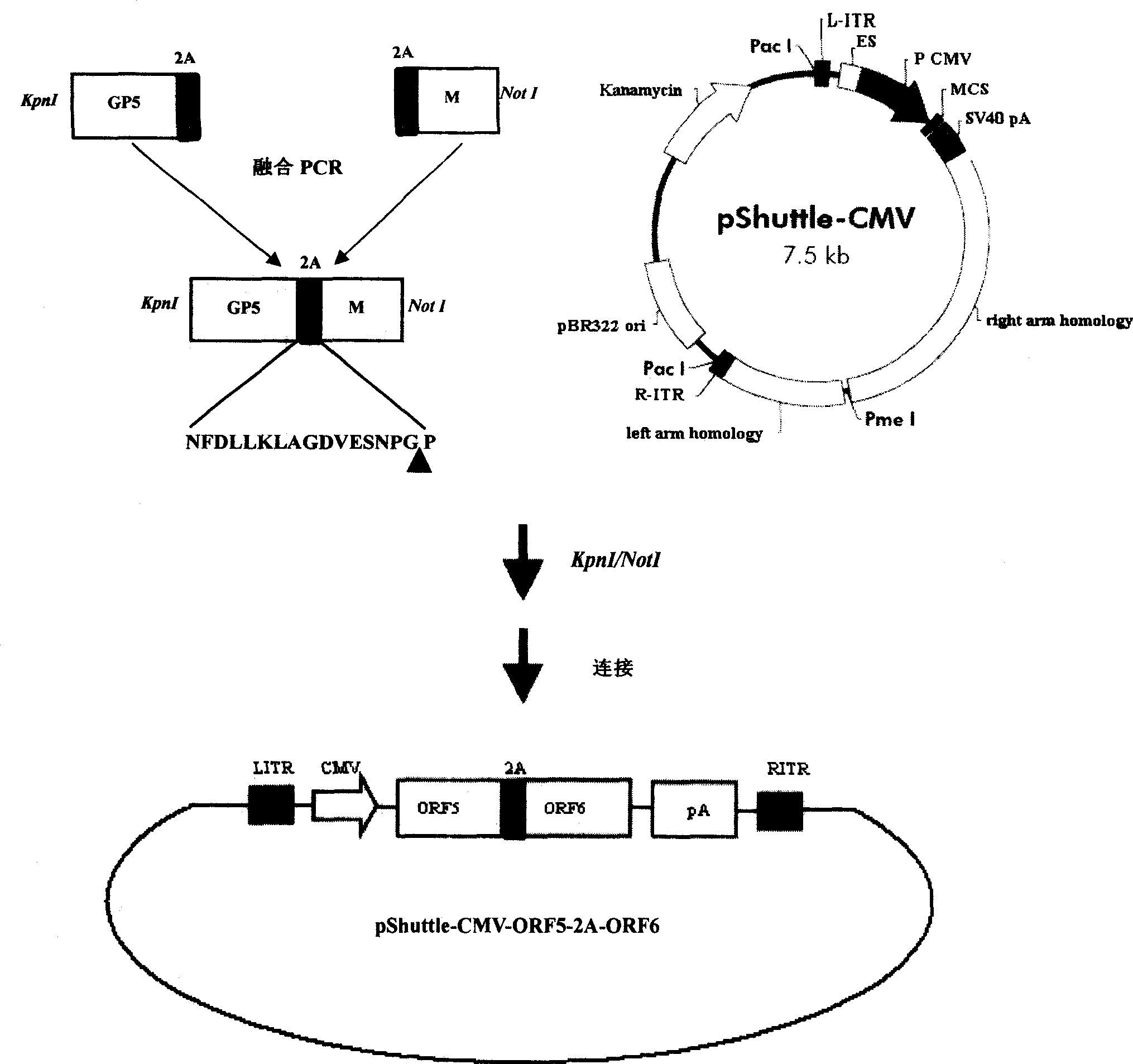
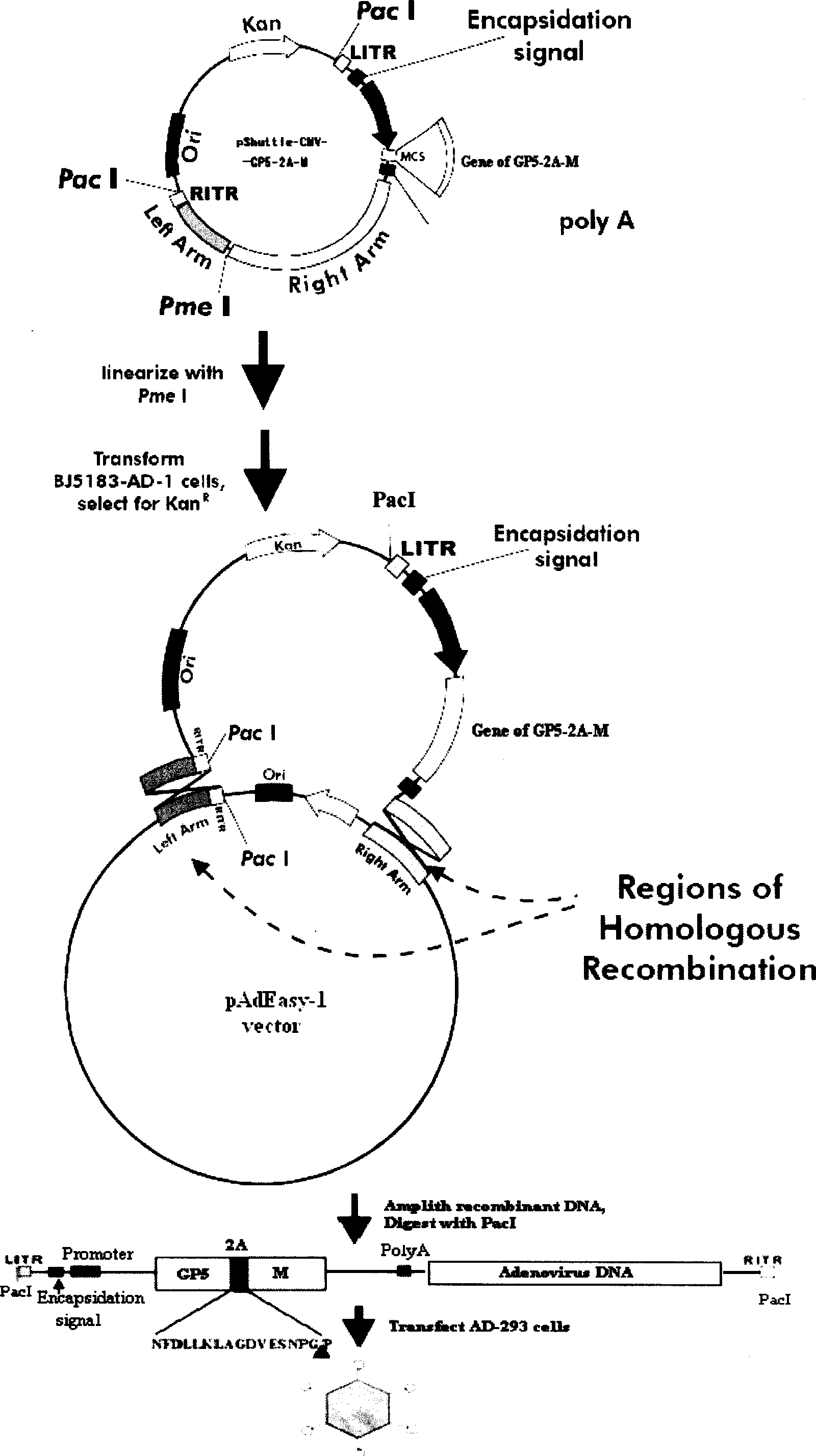
The invention discloses a porcine reproductive and respiratory syndrome divalent recombination adenovirus vaccine and the preparation method thereof. The invention belongs to the technical field of biological vaccine preparation. The vaccine can be prepared by the following steps: a GP5-2A-M fusion protein gene can be constructed by inserting an FMDV2A gene with self craking between PRRSV GP5 and M protein; homologous recombination is carried out on the GP5-2A-M fusion protein gene and adenovirus backbone plasmid pAdEasy-1; recombination adenovirus rAd-GP5-2A-M is prepared by restriction enzyme and HEK-293A cells transfection, and the divalent recombination adenovirus vaccine is prepared by the technology and the steps such as purification, amplification, and the like. After expression, the aggregate protein GP5-2A-M constructed by the invention is self cracked into GP5 and M protein, as well as exerts the viral neutralization of GP5 and the immune function of the M protein; the vaccine has stable titer with the virulent valence being 10<10.43>TCID<50> / 1.0ml, as well as has both the duplication characteristic of a routine attenuated vaccine and the safety of an inactivated vaccine; the divalent recombination adenovirus vaccine can be popularized in and applied to the control work of porcine reproductive and respiratory syndrome.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Host cell specific binding molecules capable of neutralizing viruses and uses thereof
ActiveUS20100303801A1Avoid adjustmentInhibiting and reducing spreadSugar derivativesAntiviralsCell specificViral infection
The present invention provides human binding molecules specifically binding to a host cell protein and having virus neutralizing activity, nucleic acid molecules encoding the human binding molecules, compositions comprising the human binding molecules and methods of identifying or producing the human binding molecules. The human binding molecules can be used in the diagnosis, prophylaxis and / or treatment of viral infections.
Owner:JANSSEN VACCINES & PREVENTION BV
EV71 virus neutralization epitope detection kit or reagent and preparation method thereof
The invention relates to an EV71 virus neutralization epitope detection kit or a reagent and a preparation method thereof. Particularly, the invention performs quantitative detection on EV71 virus antigen by adopting an HD6 monoclonal antibody with spectral activity. The kit or the reagent has the advantages of easy operation, high sensitivity and the like.
Owner:SINOVAC BIOTECH
Porcine circovirus II-type (PCV2) epitope peptide vaccine and preparation method thereof
ActiveCN103536912AStrong specificityEasy to saveViral antigen ingredientsAntiviralsDiseaseCircovirus
The invention relates to a porcine circovirus II-type (PCV2) epitope peptide vaccine and a preparation method of the vaccine. The vaccine contains three b cell epitopes, the lysine of the vaccine is four-branch peptide with the same core matrix structure epitope monomer and is connected with a general T ancillary cell (Th) epitope in series, and the vaccine has the molecular weight of 13kDa; the epitope peptide is named PCV CP98-156-228; after being vaccinated, a mice has stronger immune response, and a high potency virus neutralizing antibody can be generated. The epitope peptide vaccine has the advantages of being low in price, safe, high in specificity and easy to store and apply, and plays an important role in preventing and controlling the porcine circovirus disease. The PCV2 epitope peptide vaccine is prepared by four steps including predicting and screening epitope peptide, designing the epitope peptide vaccine, synthesizing, purifying and authenticating the epitope peptide as well as preparing the epitope peptide vaccine; the method is easy in raw material obtaining, lower in cost and easy to control, and has better operability, thus being suitable for being popularized and applied.
Owner:CHONGQING UNIV OF TECH +2
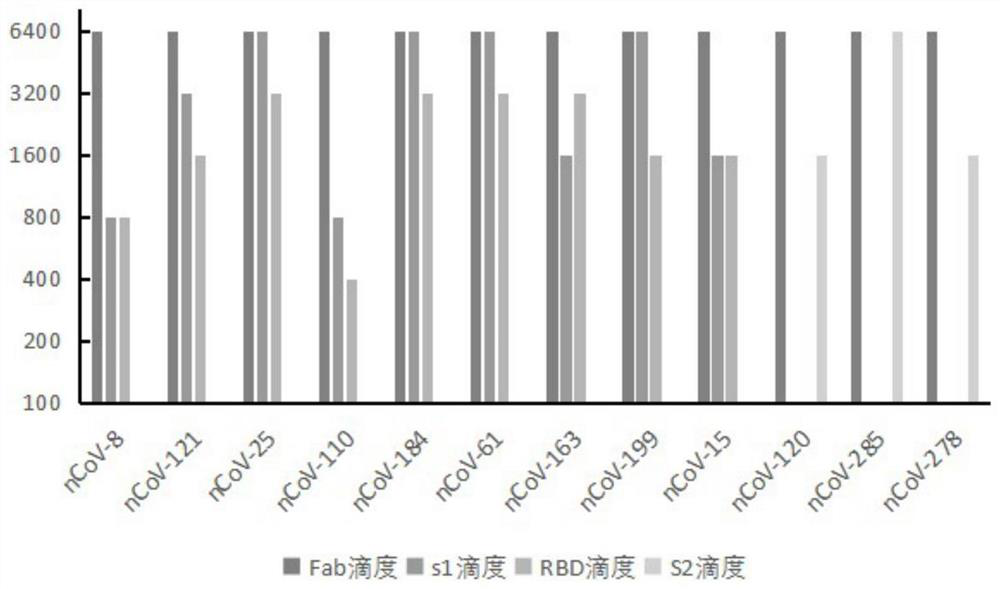
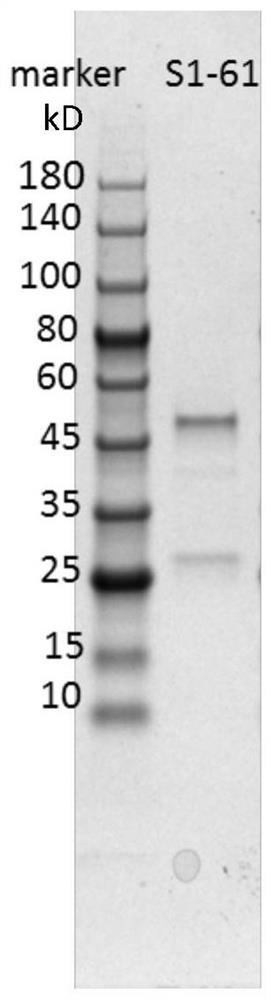
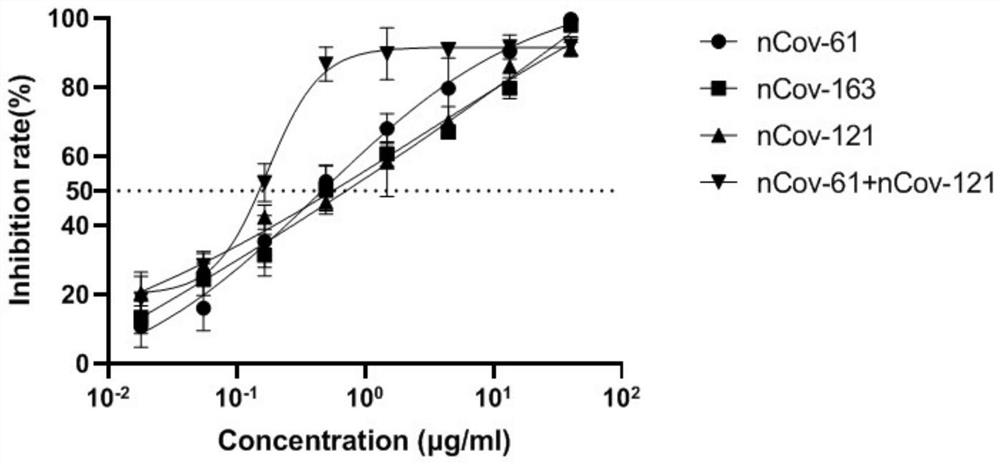
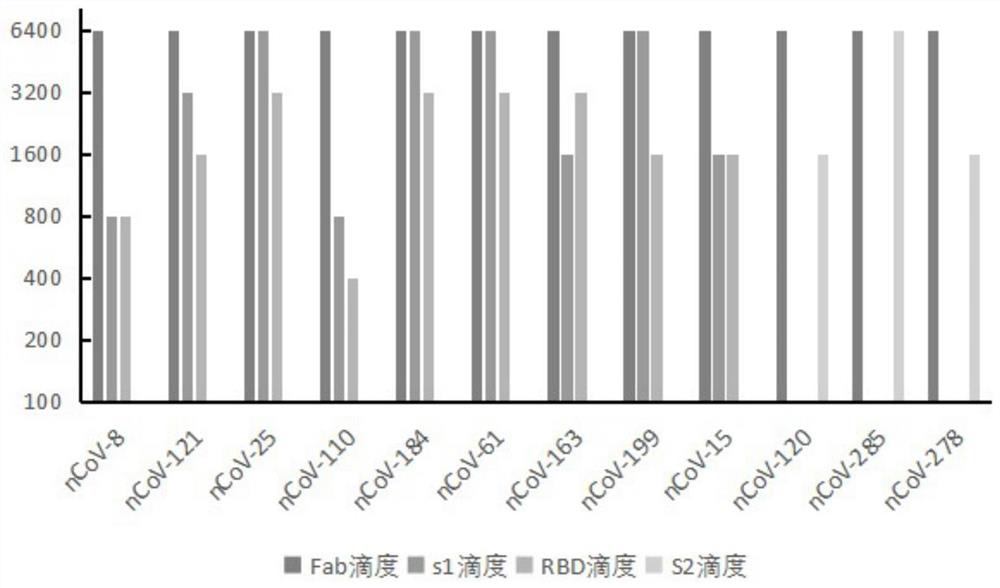
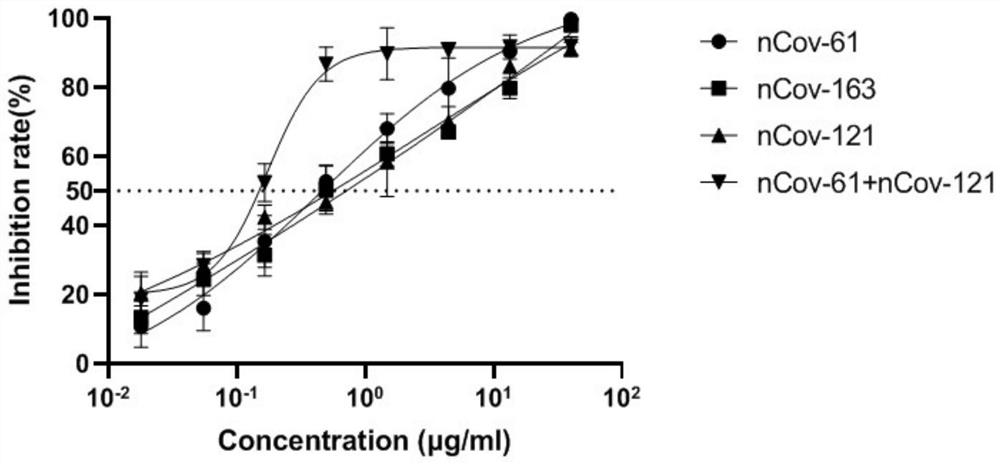
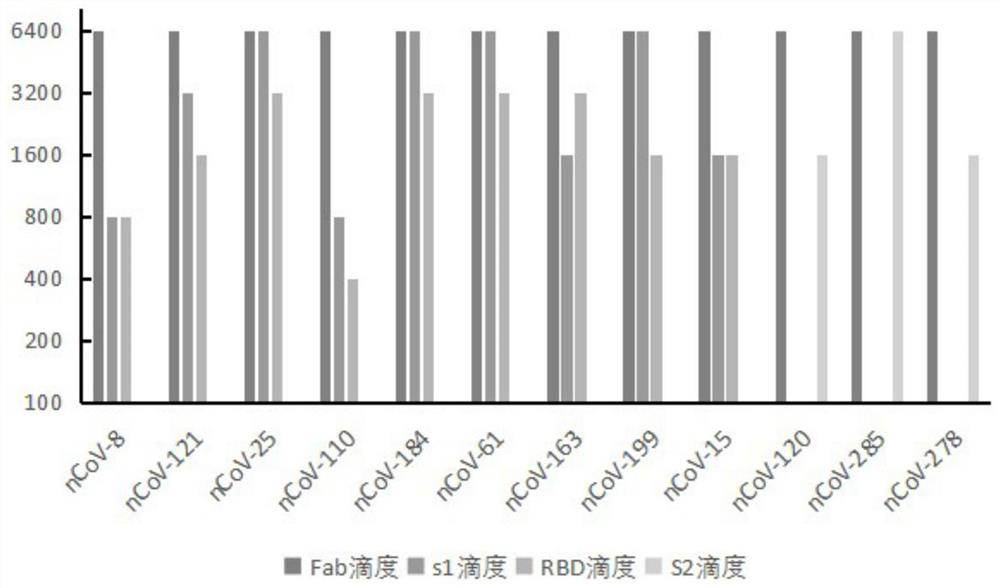
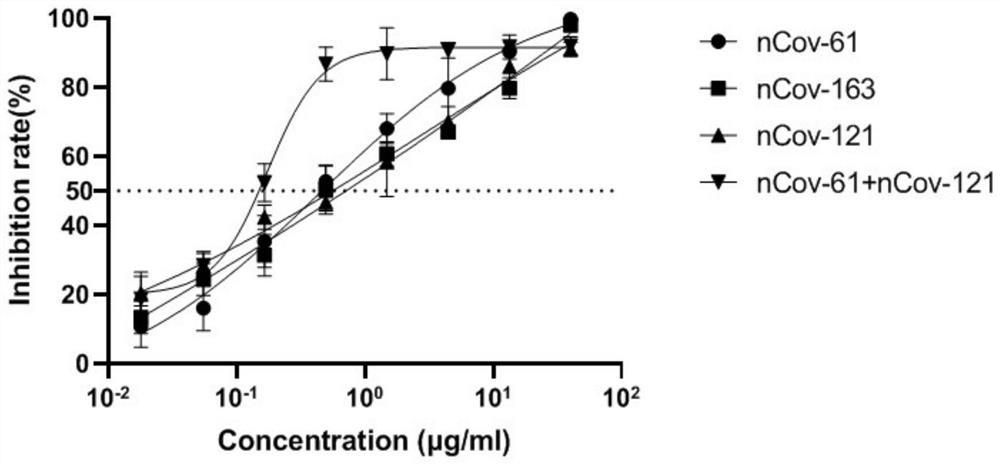
Humanized anti-novel coronavirus neutralizing antibody nCoV-61 and application thereof
ActiveCN112430265AHigh affinityImmunoglobulins against virusesAntiviralsPhage antibodiesNeutralizing antibody
The invention discloses a humanized anti-novel coronavirus neutralizing antibody nCoV-61 and an application thereof. The humanized neutralizing antibody nCoV-61 specific to the novel coronavirus surface antigen is successfully obtained by utilizing a phage antibody library technology, has a neutralizing function of preventing the novel coronavirus from infecting sensitive cells in vitro, and is high in antigen affinity, and the antibody is expected to be prepared into specific antibody drugs for preventing and treating novel coronavirus pneumonia clinically.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Hybrid tumor cell strain 1D7 capable of secreting high neutralizing activity canine distemper virus monoclonal antibody
InactiveCN102618503AHigh neutralization potencyGood treatment effectMicroorganism based processesImmunoglobulins against virusesAntiendomysial antibodiesCanine distemper virus CDV
The invention relates to a hybrid tumor cell strain capable of secreting high neutralizing activity CDV (canine distemper virus) monoclonal antibody. A hybrid tumor cell strain 1D7 is selected from a hybrid tumor cell bank containing 82 strains which secrete CDV monoclonal antibody, and is used for preparing mouse ascitic antibody which has neutralizing potency up to 106 to CDV. The hybrid tumor cell strain capable of secreting high neutralizing activity CDV monoclonal antibody is high in neutralizing potency and resistant to CDV strains in a wide range, and has evident treatment effect on CDattached dogs, minks or foxes in clinical treatment, and is safe without adverse side effects. The monoclonal antibody therapeutic agent prepared with the hybrid tumor cell strain has stable neutralizing potency after two years of storage, and is high in stability.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
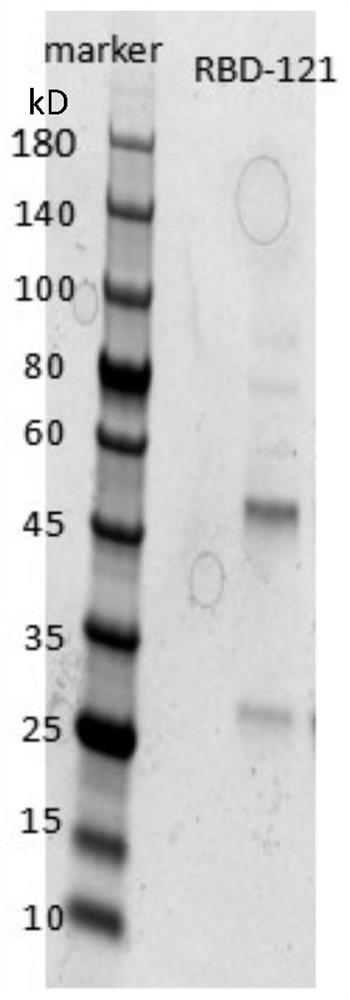
Humanized anti-novel coronavirus neutralizing antibody nCoV-121 and application thereof
ActiveCN112409479AHigh affinityImmunoglobulins against virusesAntiviralsPhage antibodiesBacteriophage
The invention discloses a humanized anti-novel coronavirus neutralizing antibody nCoV-121 and application thereof. The humanized neutralizing antibody nCoV-121 specifically aiming at a novel coronavirus surface antigen is successfully obtained with a phage antibody library technology, has a neutralizing function of preventing the novel coronavirus from infecting sensitive cells in vitro and is high in antigen affinity, thereby being expected to be prepared into specific antibody drugs for preventing and treating novel coronavirus pneumonia clinically.
Owner:中国疾病预防控制中心病毒病预防控制所
Novel coronavirus SARS-CoV-2 mRNA vaccines and preparation method and application thereof
ActiveCN113151312AProlong half-lifeEasy to getSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationSpecific igg
The invention provides novel coronavirus SARS-CoV-2mRNA vaccines and a preparation method and application thereof. The invention provides three mRNA vaccines, namely RBD, S1 and S vaccines. The RBD vaccine disclosed by the invention can induce a high-titer antigen-specific IgG antibody and a virus neutralization antibody after immunization with one dose, the high-titer neutralization antibody can be maintained for at least 26 weeks, and remarkable immune protection can be provided for human ACE2 transgenic mice in serum adoptive transfer protection experiments. The RBD and S vaccine disclosed by the invention can induce immune protection capable of completely resisting SARS-CoV-2 virus infection in the human ACE2 transgenic mice after immunization with two doses. A large number of experimental results show that the mRNA vaccine provided by the invention has good immunogenicity, forms powerful immune protection after immunizing an organism, and has a huge development potential.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Hybridoma cell strain for stable secretion of anti-PEDV monoclonal antibody and secreted antibody and application thereof
ActiveCN106380515ABiological material analysisImmunoglobulins against virusesMicroorganismBiological property
The invention discloses a hybridoma cell strain for stable secretion of anti-PEDV monoclonal antibody and the secreted antibody and application thereof. The hybridoma cell strain capable of stably secreting the anti-PEDV monoclonal antibody is named as 1B9, and the microbial preservation number is CGMCC No.12695. Researches prove that recognition epitope of the monoclonal antibody secreted by the hybridoma cell strain is conformational epitope. It shows through virus neutralization tests that the monoclonal antibody can effectively neutralize the strain of PEDV G2 subtype but has no neutralization activity to a strain of PEDV G1 subtype. In view of biological characteristics of the monoclonal antibody, the monoclonal antibody can be used for identification of the strains of PEDV G1 and G2 subtypes, and on this basis, a series of diagnostic methods are established. In addition, the monoclonal antibody also can be used for treating diseases caused by porcine epidemic diarrhea virus. The invention provides a novel technological means for diagnosis and treatment of PEDV G1 subtype.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Jcv neutralizing antibodies
ActiveUS20150056188A1Reduce viral loadIncrease awarenessImmunoglobulins against virusesAntibody ingredientsAntibody SuppressionAntiendomysial antibodies
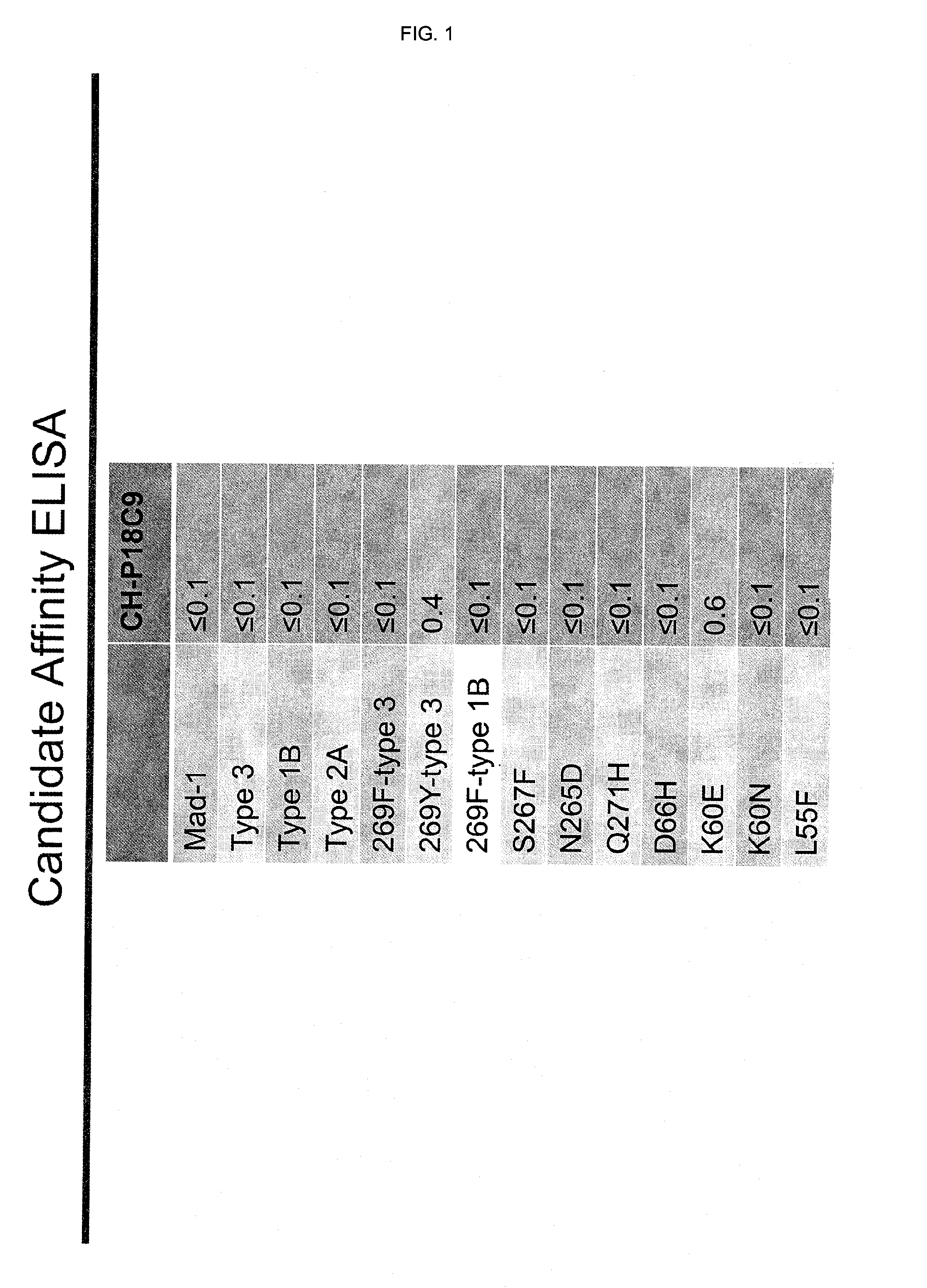
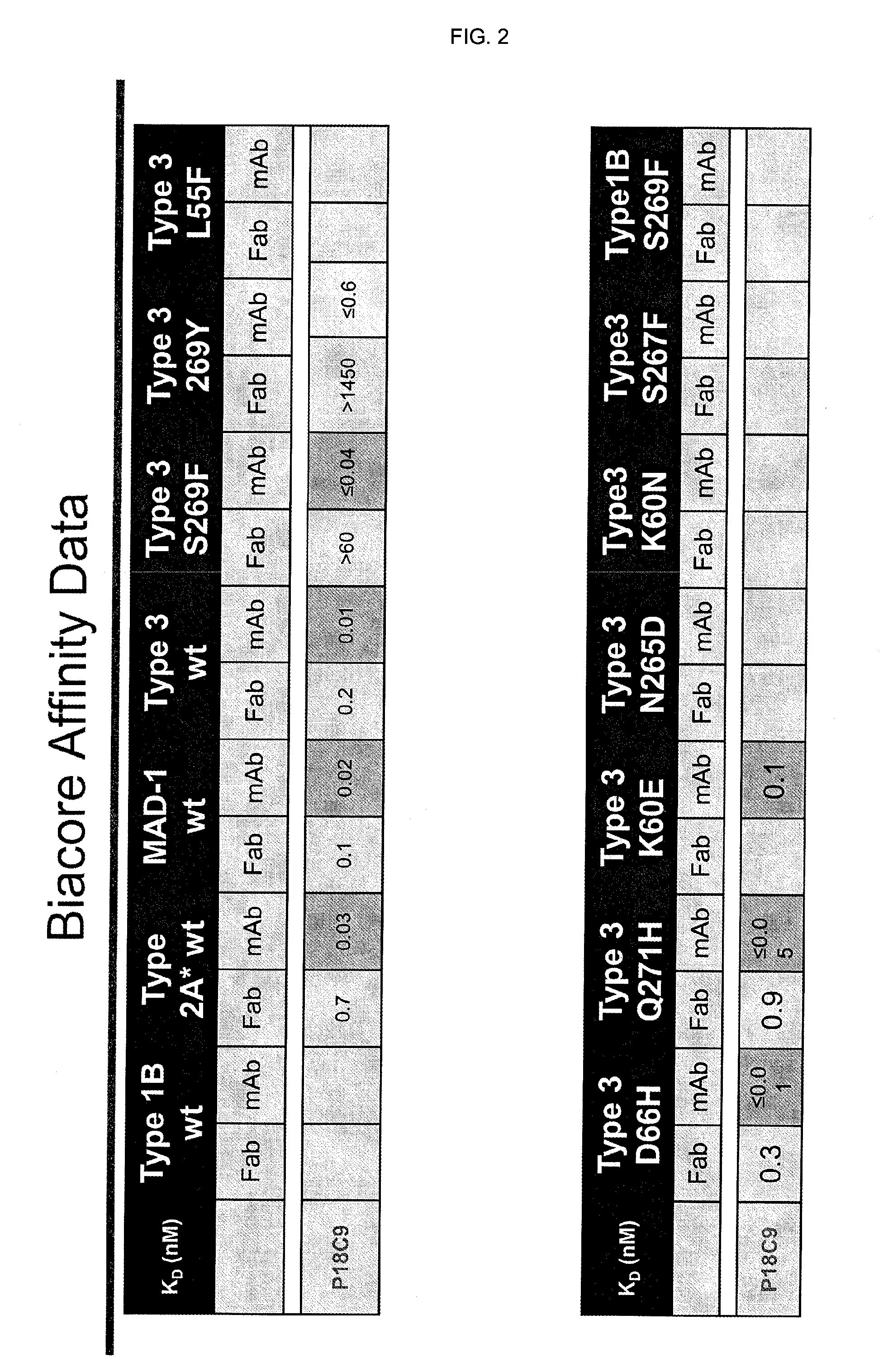
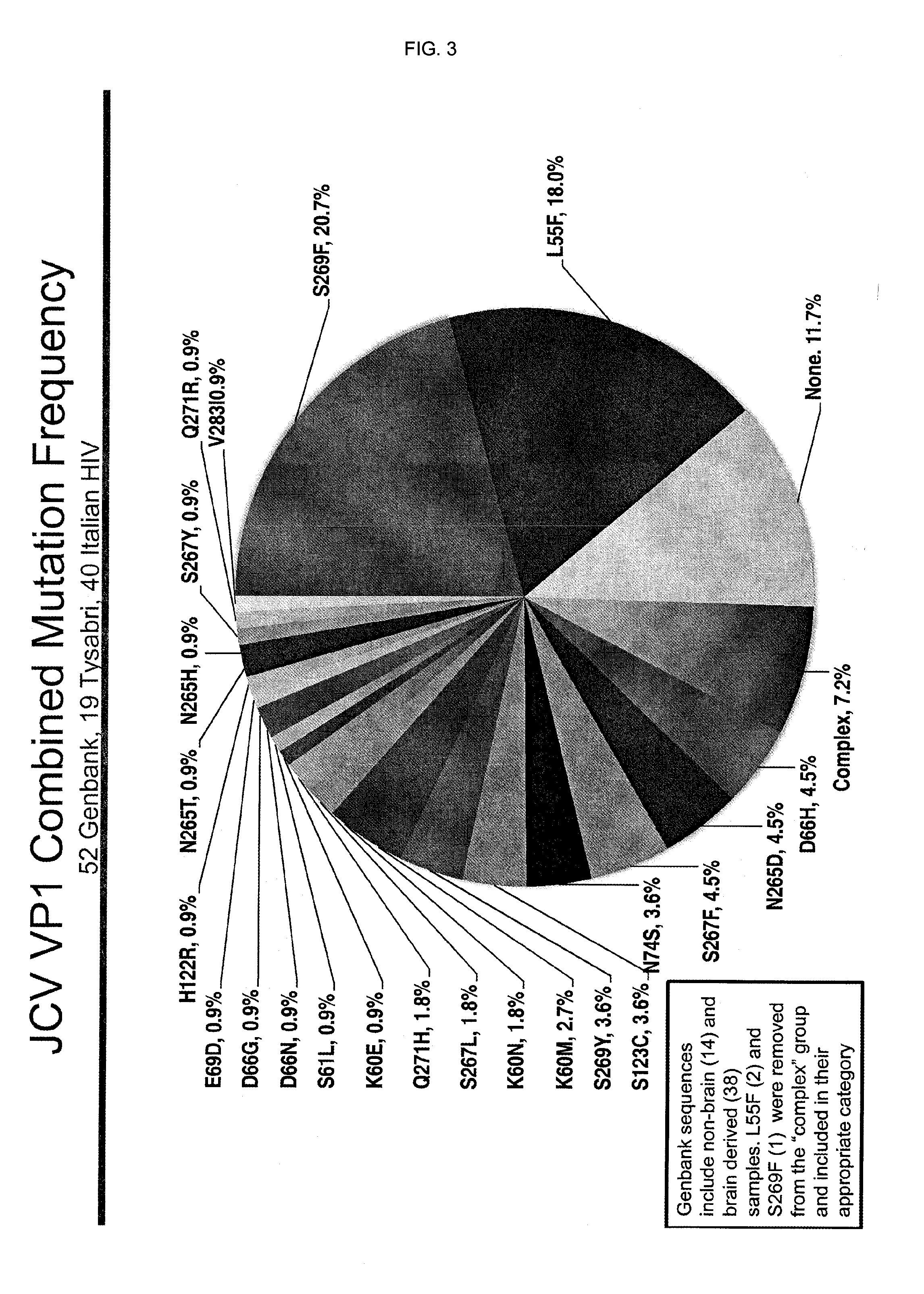
In one aspect, the disclosure provides neutralizing antibodies against JCV and methods for the treatment of PML. In some embodiments, aspects of the invention relate to an isolated JC-virus neutralizing monoclonal antibody against JCV capsid protein VPI (JCV-VP1). In some embodiments, the antibody suppresses infectivity of the JC-virus. In some embodiments, the antibody binds the sialic acid binding pocket of JCV-VPI. In some embodiments, the antibody binds JCV-VP 1 comprising one or more of the following mutations: S269F, S269Y, S267F, N265D, Q271 H, D66H, K60E, K60N and L55F.
Owner:BIOGEN MA INC
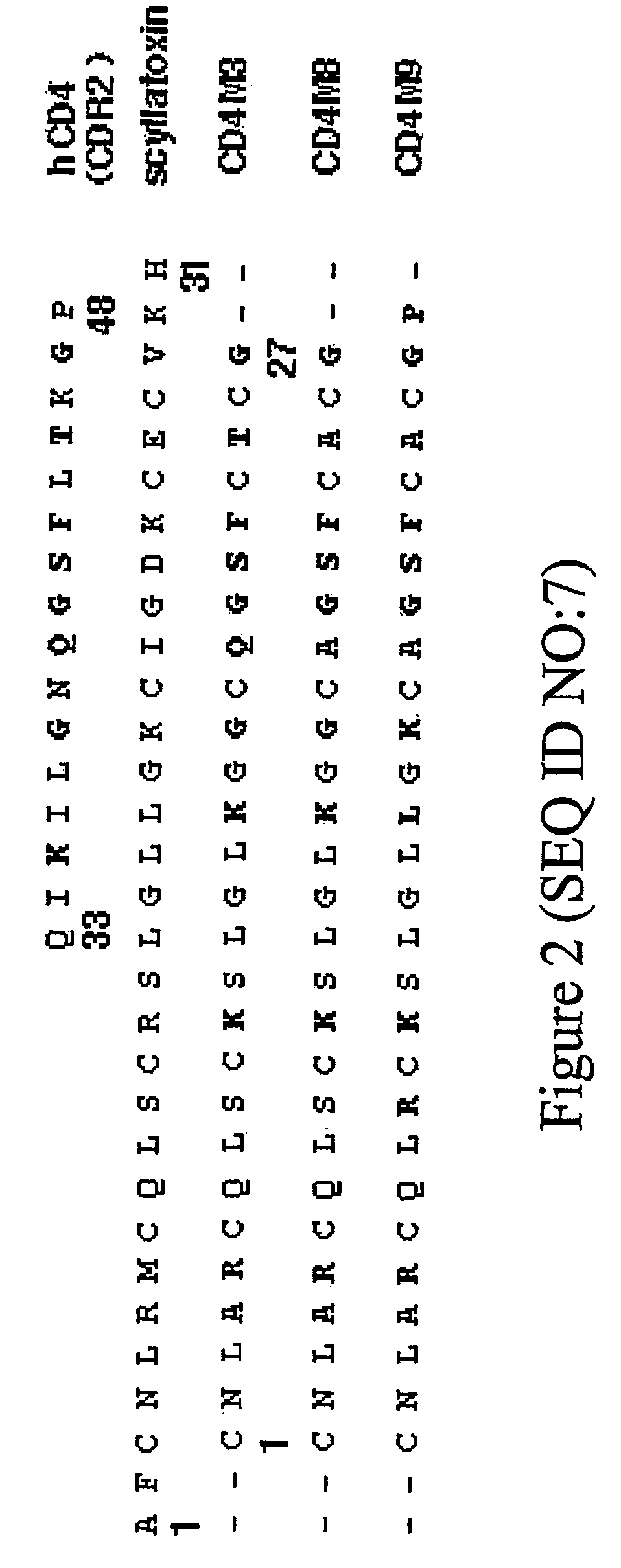
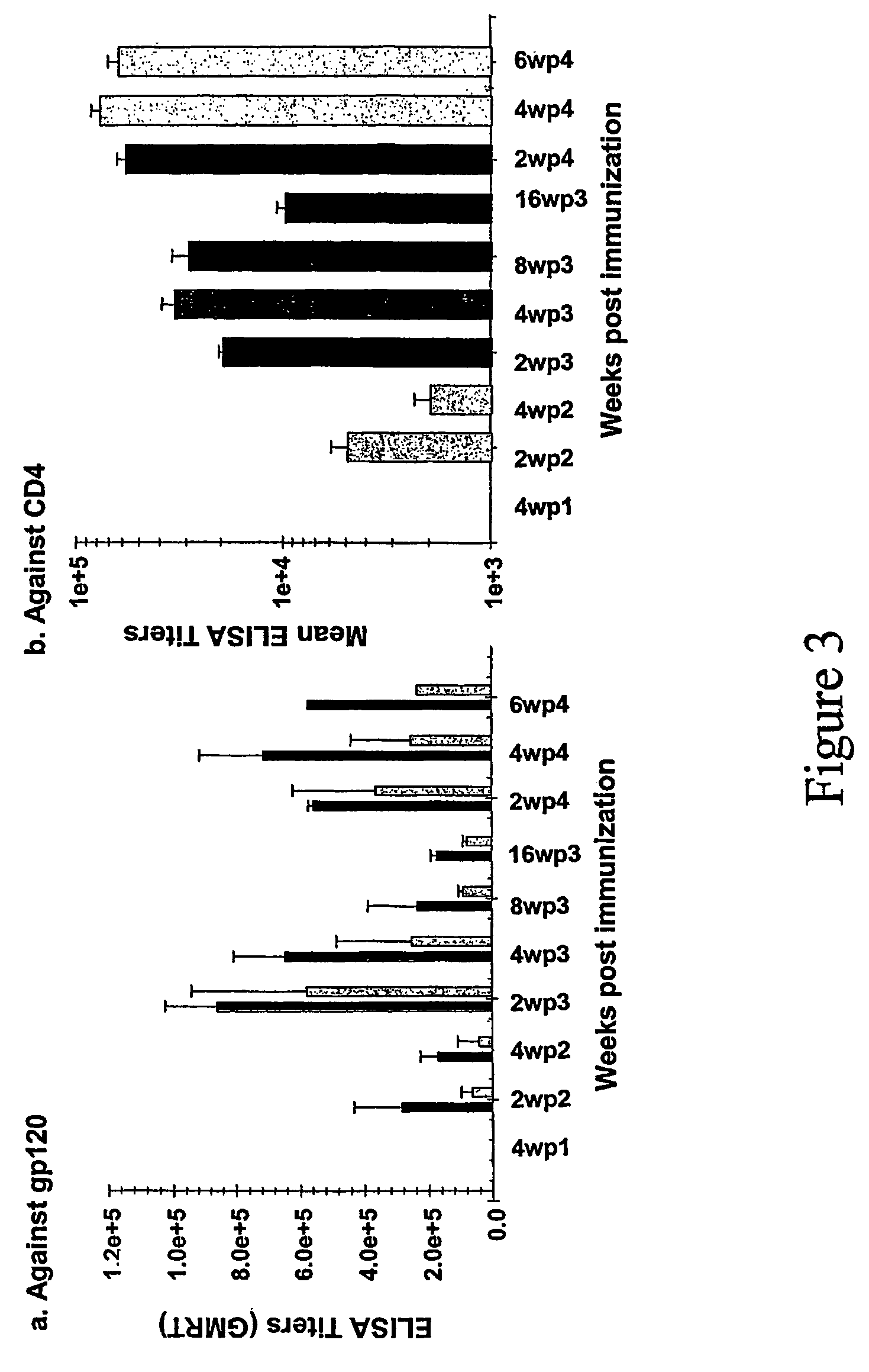
Chimeric HIV Env proteins comprising CD4 mini-proteins or CD4 mimetics that are capable of inducing neutralizing antibody responses against cryptic Env epitopes
Env-CD4 complexes and hybrids are disclosed that expose cryptic epitopes that are important in virus neutralization. Methods of diagnosis, treatment and prevention using the polynucleotides and polypeptides are also provided.
Owner:NOVARTIS AG +1
Humanized anti-novel coronavirus neutralizing antibody nCoV-163 and application thereof
ActiveCN112341541AHigh affinityImmunoglobulins against virusesAntiviralsPhage antibodiesPharmaceutical drug
The invention discloses a humanized anti-novel coronavirus neutralizing antibody nCoV-163 and application thereof. According to the humanized anti-novel coronavirus neutralizing antibody nCoV-163 andthe application thereof, the humanized neutralizing antibody nCoV-163 specifically aiming at a novel coronavirus surface antigen is successfully obtained by utilizing a phage antibody library technology, the antibody nCoV-163 is provided with a neutralizing function of preventing novel coronavirus from infecting sensitive cells in vitro and is high in antigen affinity, and the antibody is expectedto be prepared into specific antibody drugs for preventing and treating novel coronavirus pneumonia clinically.
Owner:中国疾病预防控制中心病毒病预防控制所
Hiv envelope-cd4 complexes and hybrids
Env-CD4 complexes and hybrids are disclosed that expose cryptic epitopes that are important in virus neutralization. Methods of diagnosis, treatment and prevention using the polynucleotides and polypeptides are also provided.
Owner:NOVARTIS AG +1
Competitive enzyme linked immunosorbent assay (C-ELISA) for the detection of a flavivirus specific antibody
InactiveCN101449165ASsRNA viruses positive-senseBiological material analysisSpecific immunitySecondary Infections
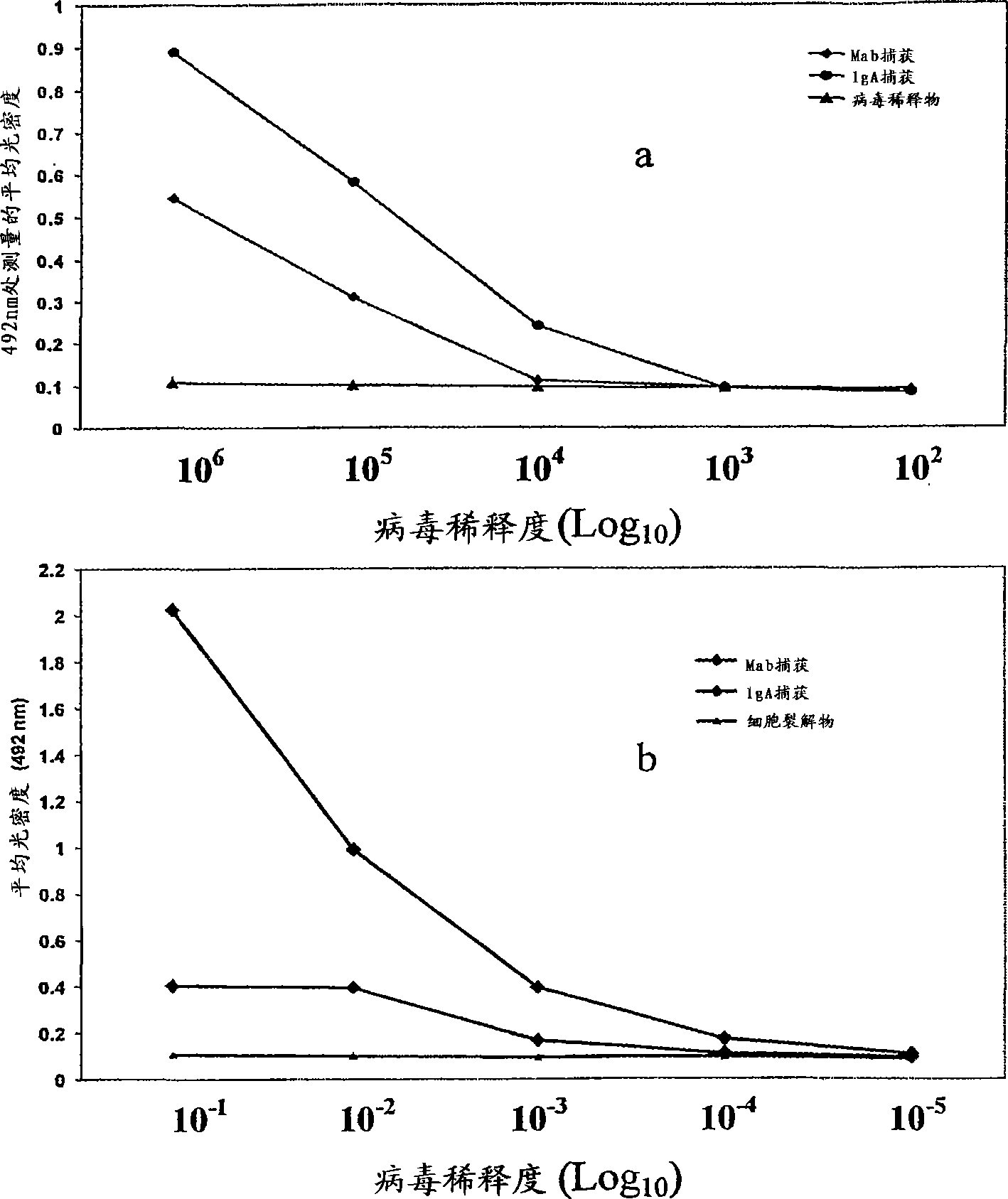
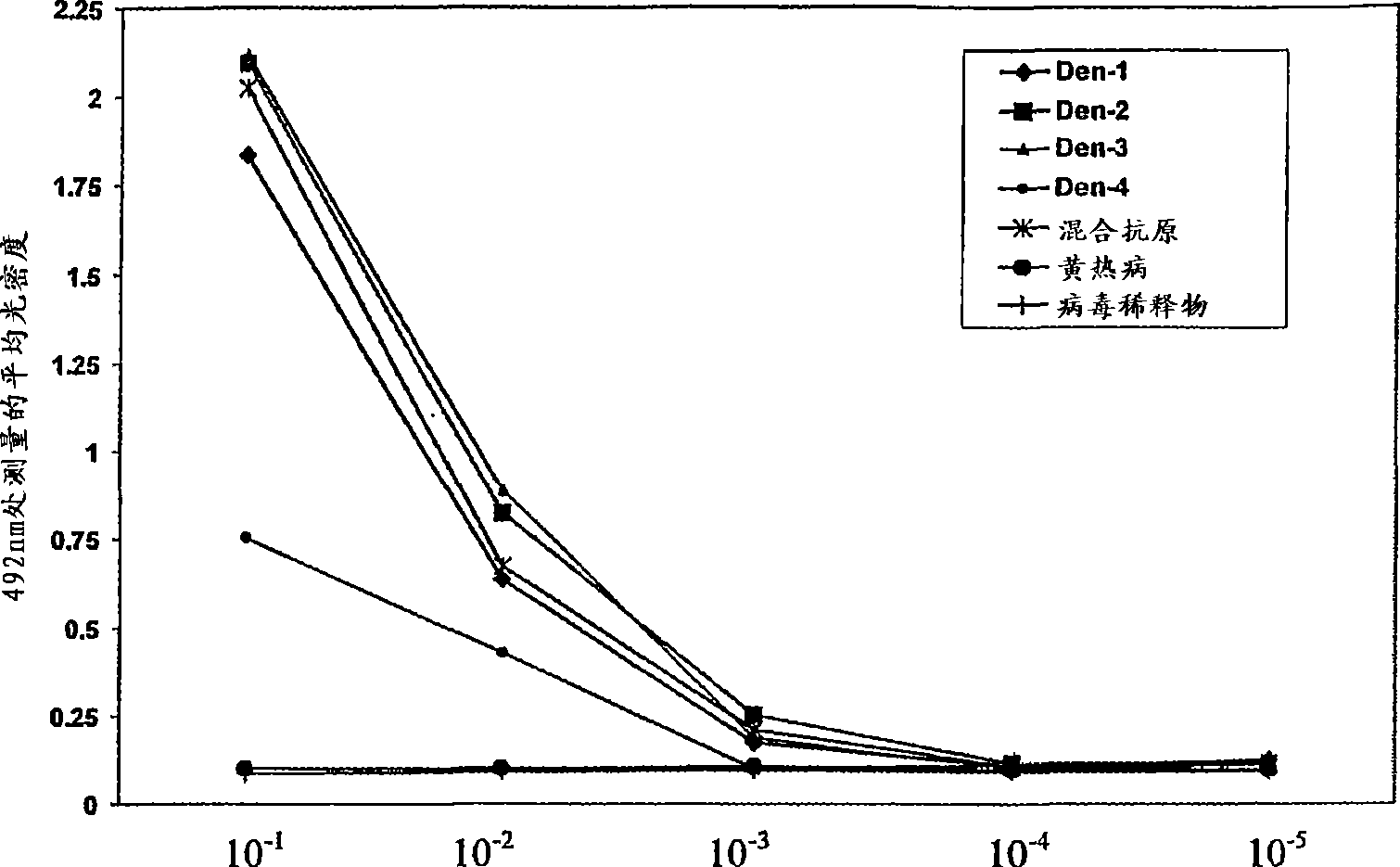
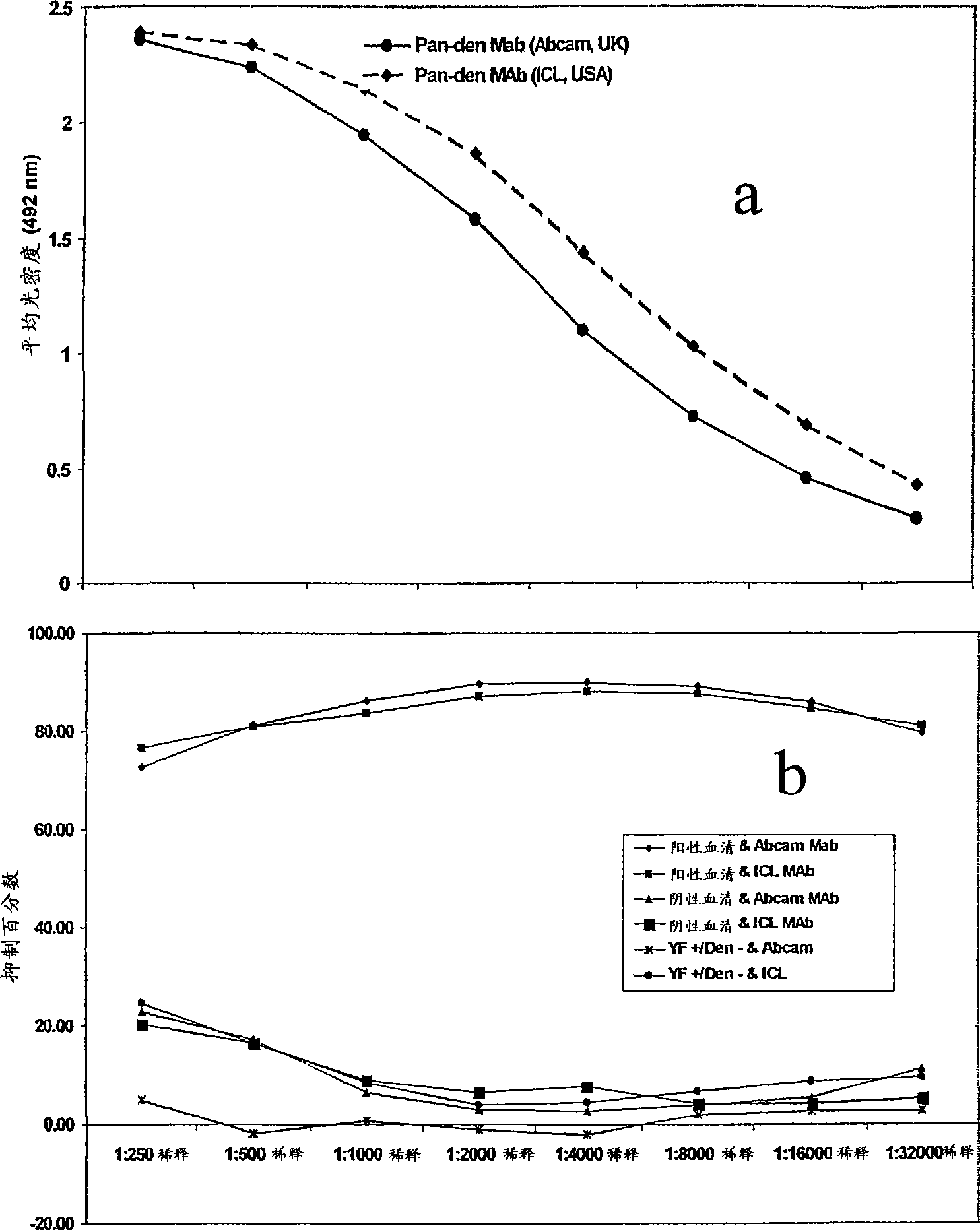
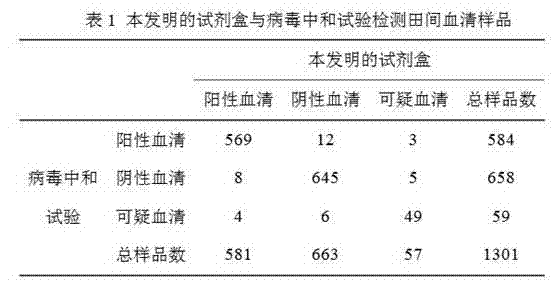
A competitive enzyme-linked immunosorbent assay (C-ELISA), using flavivirus member specific immunological agents was developed to detect antibody specific to members of the flaviviruses indicative of exposure to flavivirus. The test is based on a competition for epitope binding on the envelope protein of the flavivirus antigen captured using anti-flavivirus IgA in the presence of flavivirus positive serum. This test has comparable sensitivity specificity and speed to the virus neutralization assay (VNT). C-ELISA is a versatile technique, which could have various applications. Slight modifications of this protocol could lead to a C-ELISA-based detection method of secondary infection or one that could be used for serotype specific sero-epidemiological studies and / or vaccine evaluation. The protocol developed for C-ELISA was demonstrated using dengue lysate antigen and dengue specific monoclonal antibody. This can be used against other flaviviruses and the results for Japanese encephalitis illustrates this.
Owner:NATIONAL ENVIRONMENT AGENCY
BEFV (Bovine Ephemeral Fever Virus) indirect ELISA (Enzyme Linked Immunosorbent Assay) antibody detection kit and preparation method thereof
ActiveCN103969435AReactiveGuaranteed specificityBiological material analysisBovine ephemeral feverEpidemiologic survey
The invention relates to an indirect ELISA (Enzyme Linked Immunosorbent Assay) kit for detecting a BEFV (Bovine Ephemeral Fever Virus) serum antibody, as well as a preparation method of BEFV recombinant antigen used in the kit and the recombinant antigen. The recombinant protein only reacts with the BEFV antibody, and does not generate cross reaction with antibodies for other viruses in the same family, so that the recombinant protein has extremely high specificity; and the recombinant protein also has good reaction sensitivity. In addition, the repeatability of the kit is good, virus neutralization tests have high coincidence rate, and the storage life is long. The technical requirements are low during the operation of the kit, and the kit can be widely promoted and used in production, and applied to the BEFV epidemiological investigation and immune level monitoring.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Enzyme-linked immuno sorbent assay (ELISA) kit for detecting sheep pseudo rabies virus (PRV) antibody
ActiveCN104181297AStrong specificityIncreased sensitivityBiological material analysisPositive controlElisa kit
The invention relates to the technical field of animal epidemic disease diagnosis, and in particular relates to an enzyme-linked immuno sorbent assay (ELISA) kit for detecting a sheep pseudo rabies virus (PRV) antibody. The ELISA kit comprises an envelop plate, a positive control, a negative control, sample diluent, an enzyme label conjugate, 20*concentration washing liquid, substrate liquid, stop buffer, sealing glue, a self-sealing bag and an operation instruction; the kit is capable of rapidly, sensitively and accurately detecting the PRV antibody in the body of a sheep, so that the blank in the field at home and abroad is made up for. The kit can be used for replacing traditional serological methods such as a virus neutralization test, so that the high-flux and large-scale detection on the PRV antibody in the body of the sheep, with the high sensitivity, strong specificity and good stability, can be realized.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
SARS-CoV-2 surrogate virus neutralization assay test kit
Owner:NAT UNIV OF SINGAPORE
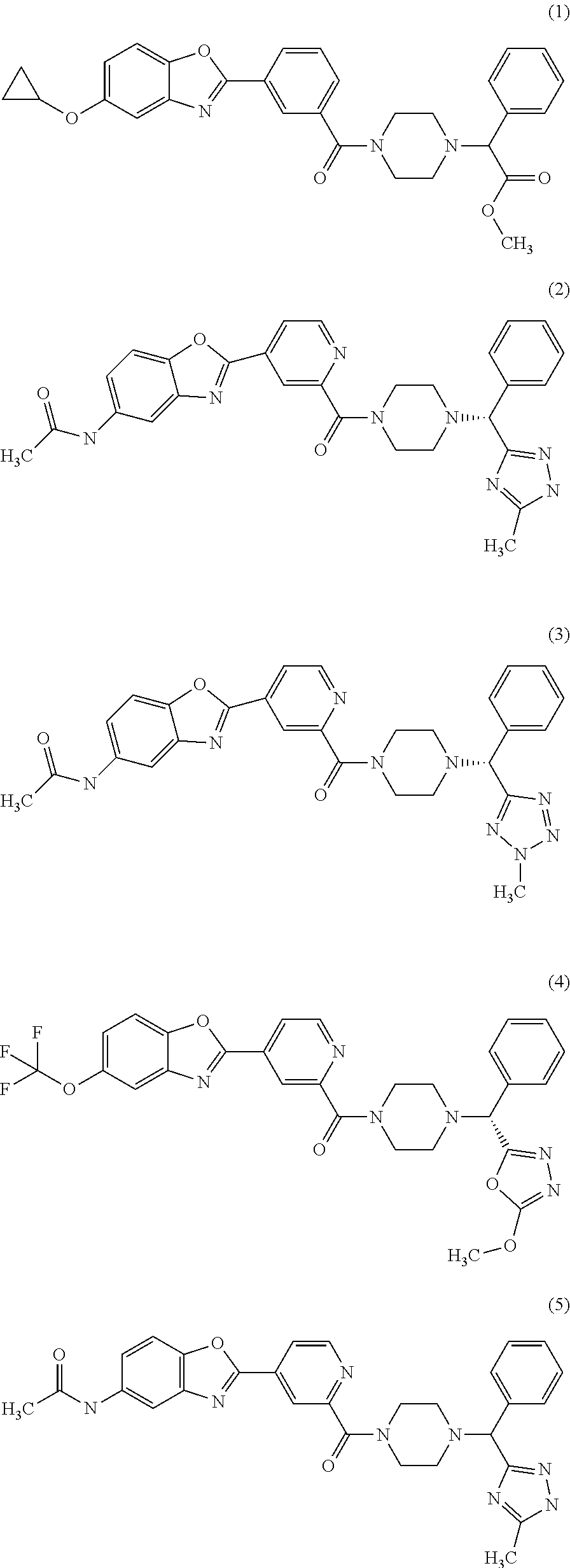
Piperazine derivatives for influenza virus inhibitions
ActiveUS20200010459A1Neutralizing activityCompetitive binding activityOrganic chemistryAntiviralsHemagglutininPharmaceutical drug
The present invention provides piperazine derivatives exhibiting high affinity to the stem region (viral membrane proximal part) of influenza hemagglutinin as determined through competition binding and high virus neutralization activity while having low cytotoxity. Furthermore, the present invention relates to pharmaceutical compositions comprising said piperazine derivatives, methods of preparing said piperazine derivatives, as well as said piperazine derivatives for use in medical prevention or treatment, especially for preventing or treating influenza.
Owner:JANSSEN VACCINES & PREVENTION BV
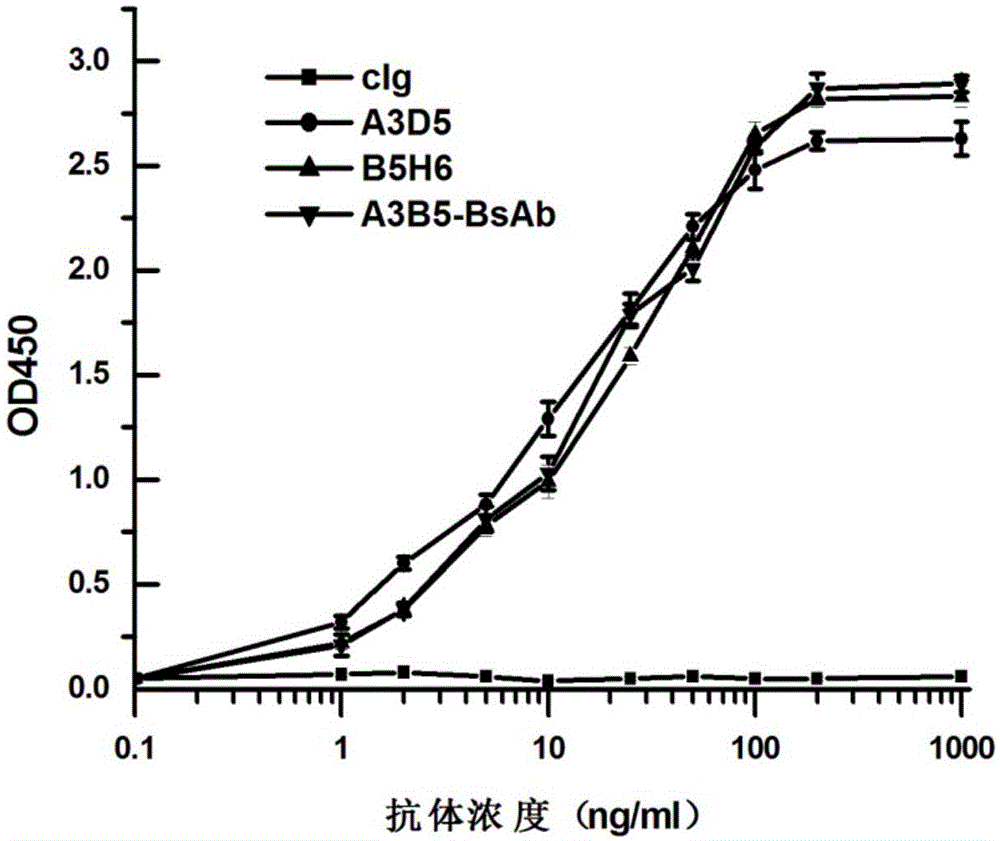
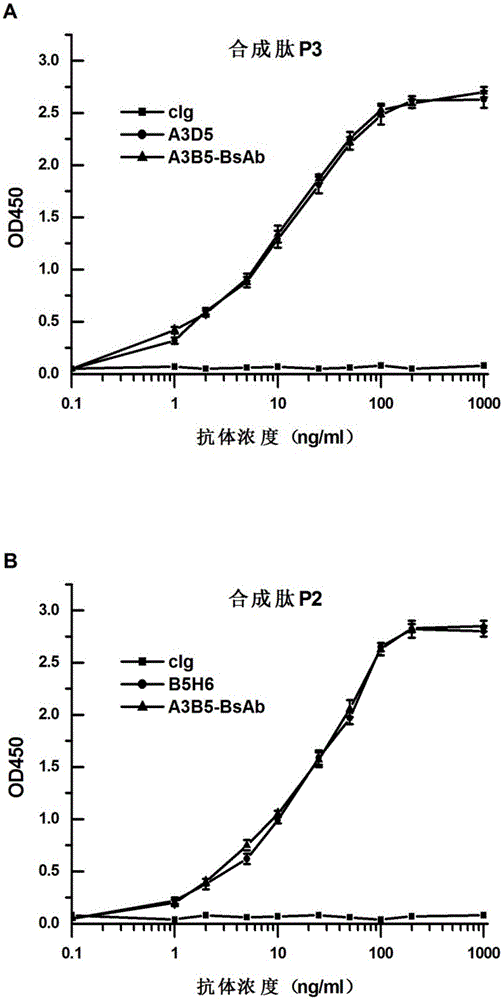
Bispecific antibody for hepatitis B surface protein, and use thereof
ActiveCN105061590AAvoid infectionCombined Application ExcellenceImmunoglobulins against virusesAntiviralsHepatitis a+b vaccineHumanized antibody
The invention provides a bispecific antibody A3B5-BsAb for a hepatitis B surface protein. The bispecific antibody is formed by combining an antibody A3D5 with an antibody B5H6. The bispecific antibody has better HBV virus neutralization ability and HBsAg release inhibition ability than single use of the antibody A3D5 and the antibody B5H6, and has strong synergistic effects, so the bispecific antibody is likely to prevent HBV infection related hepatitis, hepatic cirrhosis and liver cancer. The bispecific antibody is a completely humanized antibody obtained through cloning HBsAg specific memory B cells in the peripheral blood of hepatitis B vaccine inoculated volunteer, has lower immunogenicity than murine, chimeric and humanized antibodies, and can be used to prepare hepatitis B virus related hepatopathy prevention or treatment drugs or diagnose reagents.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Anti-Ebola virus monoclonal antibody, preparation method and uses thereof
ActiveCN108570106AHigh ADCC activityHigh activityGenetically modified cellsImmunoglobulins against virusesEbola virusHeavy chain
The invention belongs to the field of immunology and molecular biology, and relates to an anti-Ebola virus monoclonal antibody, a preparation method and uses thereof, specifically to an anti-Ebola virus monoclonal antibody or an antigen-binding part thereof, wherein the amino acid sequences of the light chain variable region and the heavy chain variable region comprise one group selected from thefollowing groups (1)-(3): the group (1): the amino acid sequence of the light chain variable region is represented by SEQ ID NO:4, and the amino acid sequence of the heavy chain variable region is represented by SEQ ID NO:6; the group (2): the amino acid sequence of the light chain variable region is represented by SEQ ID NO:10, and the amino acid sequence of the heavy chain variable region is represented by SEQ ID NO:12; and the group (3): the amino acid sequence of the light chain variable region is represented by SEQ ID NO:16, and the amino acid sequence of the heavy chain variable region is represented by SEQ ID NO:18. According to the present invention, the anti-Ebola virus monoclonal antibody has advantages of enhanced ADCC activity, good antigen-binding activity and good virus-neutralizing activity.
Owner:BEIJING MABWORKS BIOTECH
Epitope vaccine for resisting A/B subgroup avian leucosis virus infection and preparation method and application of epitope vaccine
ActiveCN104548087ALow costEase of mass productionAntiviralsAntibody medical ingredientsLeucosisNucleotide
The invention relates to the field of animal virology and immunology, and provides an epitope vaccine for resisting A / B subgroup avian leucosis virus infection. The epitope vaccine is prepared by highly active recombinant protein His-cENV which is obtained through screening and purification after prokaryotic expression and a freund's adjuvant in a united manner, wherein the nucleotide sequence for encoding the recombinant protein His-cENV is shown as SEQ ID NO.1. Through the epitope vaccine, 7-day-old breeding poultry chicks are immune and can produce 1:128000 neutralizing antibodies. Vitro virus neutralization experiments and animal experiments show that the epitope vaccine can neutralize different ALV-A / B isolated strains, so that chicken flocks are effectively protected to resist the infection of ALV-A / B strains. Because the epitope vaccine is based on a multi-epitope antigen gene sequence which is originated form env, the defect of ALV-A / B virus variation is overcome, a new era of the ALV-A / B vaccine is opened, a new way of resisting the ALV-A / B infection is provided, and a technical support for preventing and controlling the ALV-A / B is provided.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Nano antibody for novel coronavirus and application of nano antibody
The invention discloses a nano antibody for a novel coronavirus and application of the nano antibody. Specifically, the invention provides the nano antibody for the novel coronavirus and a sequence ofthe nano antibody. The invention also provides polynucleotide coding the nano antibody, a corresponding expression vector, a host cell capable of expressing the nano antibody and a production methodof the nano antibody. The nano antibody disclosed by the invention can be specifically combined with human SARS-CoV2; the nano antibody disclosed by the invention has good ACE2 / SARS-CoV2S-RBD blockingactivity; the nano antibody disclosed by the invention can be combined with various mutants of new coronavirus; the nano antibody disclosed by the invention has relatively high neutralizing activityon SARS-CoV2 euvirus; and the antibody disclosed by the invention is stable in quality before and after being atomized.
Owner:SHANGHAI NOVAMAB BIOPHARM CO LTD
Neutralizing single-domain antibody with function of resisting novel coronavirus SARS-Cov-2 and application of neutralizing single-domain antibody
ActiveCN112062838ABiological material analysisImmunoglobulins against virusesSingle-domain antibodyNeutralizing antibody
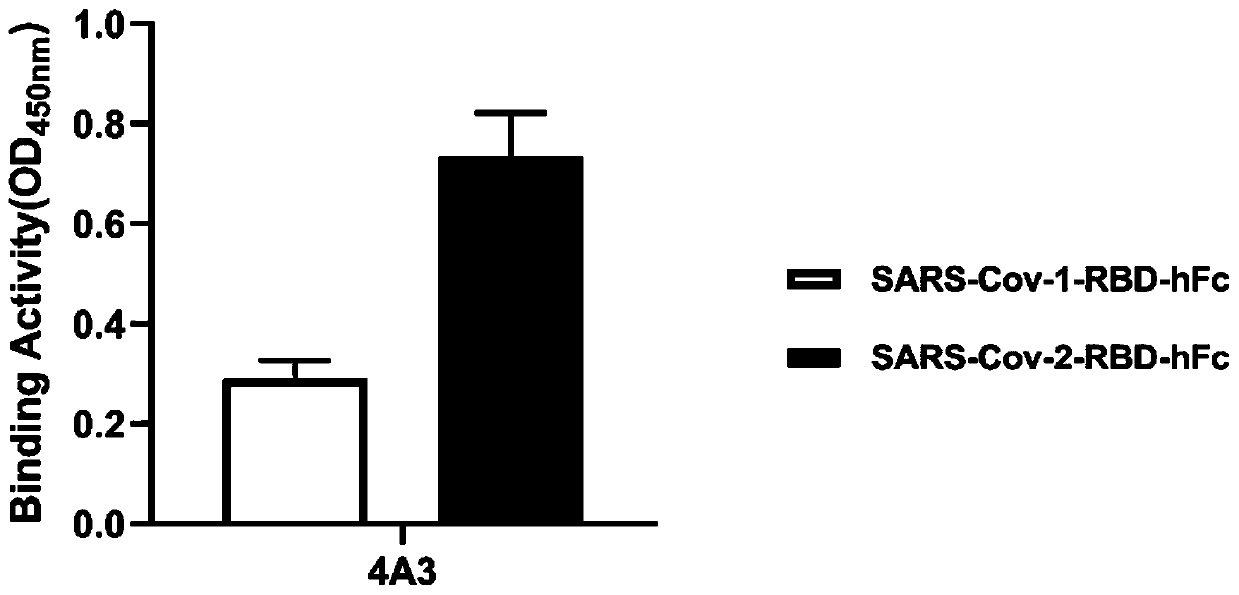
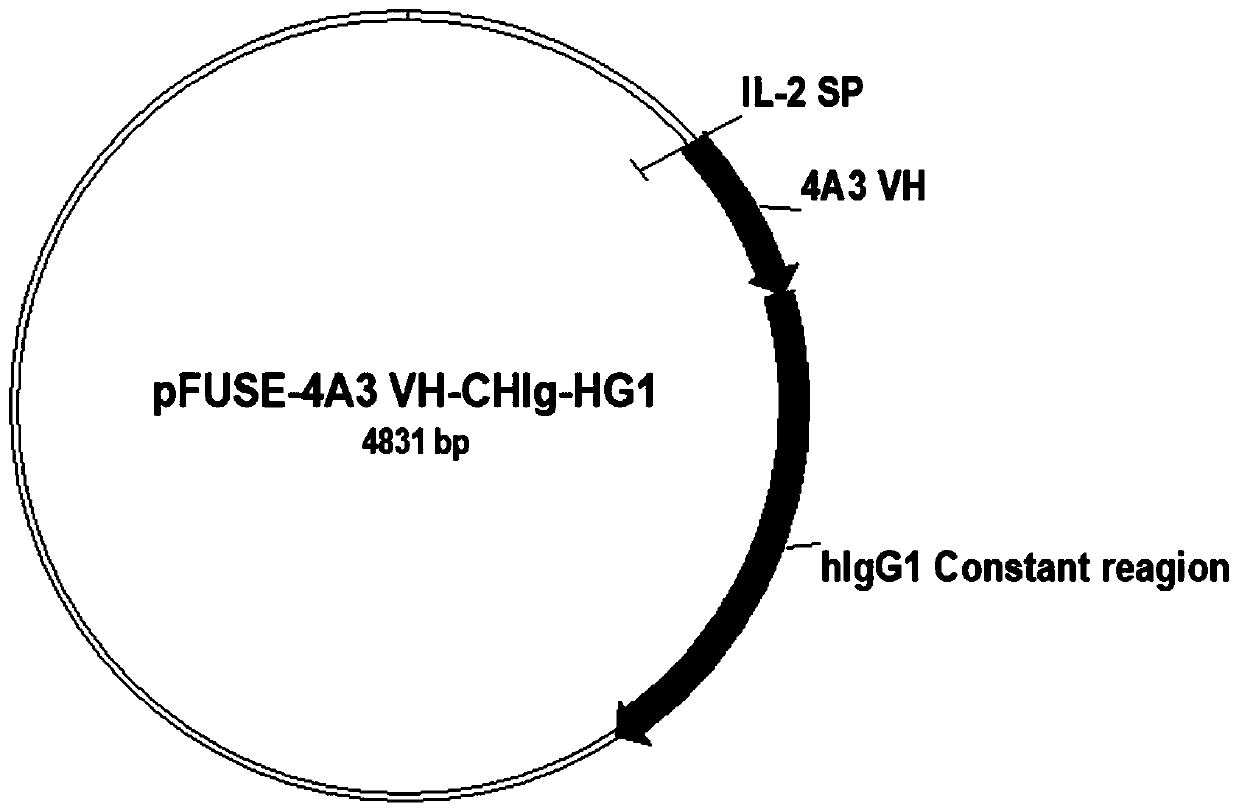
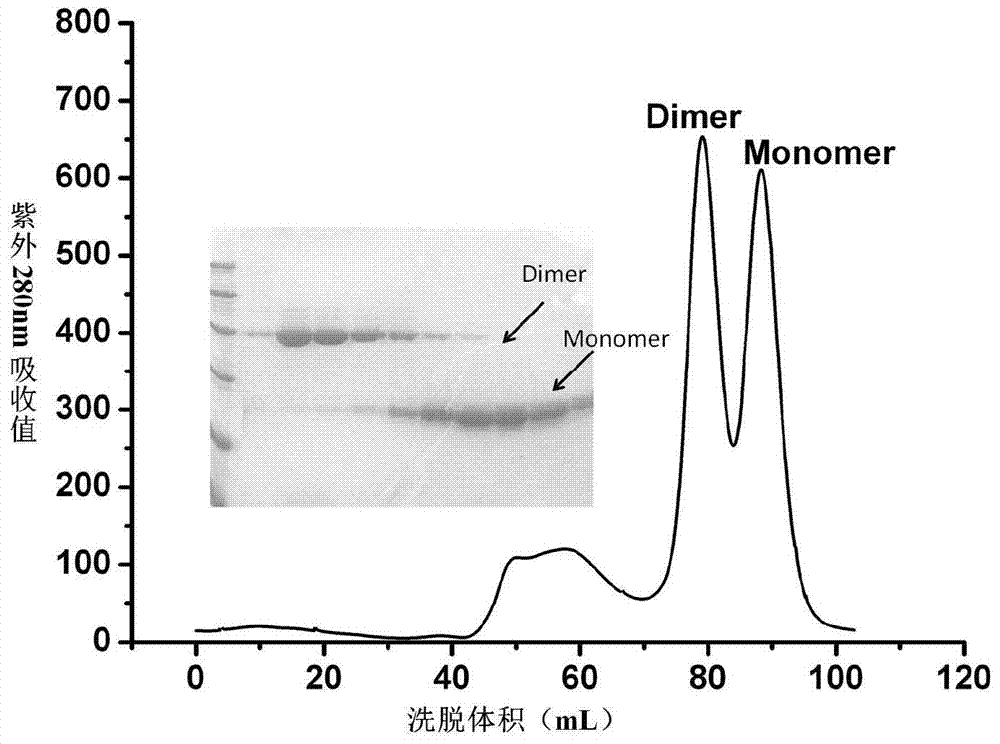
The invention relates to a neutralizing single-domain antibody with a function of resisting novel coronavirus SARSCov2 and application of the neutralizing single-domain antibody. The antibody has at least one of a heavy chain CDR1, a heavy chain CDR2 and a heavy chain CDR3. The antibody can be used for preparing a diagnostic reagent or a diagnostic kit, an antibody medicine or a medicinal composition against COVID-19. An SARS-CoV-2 specific human monoclonal single-domain antibody is obtained by a phage display technology, has an SARS-CoV-2 pseudo-virus neutralizing effect, and has a synergistic neutralizing effect on D614G mutant strain pseudovirus when being combined with an IgG type SARS-CoV-2 neutralizing antibody 4A3 for use. Therefore, the antibody provides an efficient alternative antibody medicine for prevention and treatment of COVID-19.
Owner:NANJING MEDICAL UNIV
Novel coronavirus neutralizing antibody detection kit
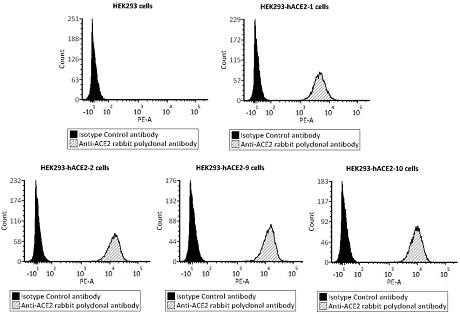
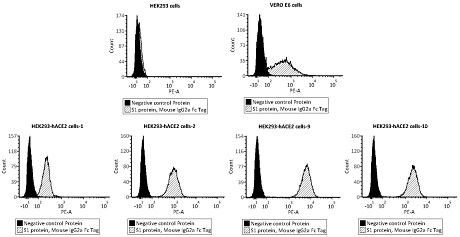
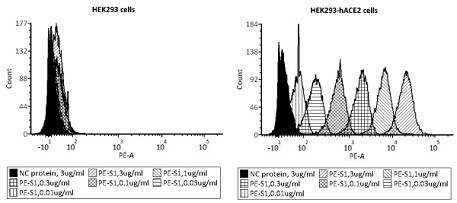
ActiveCN112098660AReduce dosageReduce stepsBiological testingImmunoassaysVirus NeutralizationNeutralizing antibody
The invention provides a novel coronavirus neutralizing antibody detection kit. The kit comprises HEK293-hACE2 cells, PE labeled novel coronavirus spike glycoprotein S1, a novel coronavirus neutralizing antibody positive standard substance, a negative control antibody sample and a flow detection buffer solution. Also provided is a method for detecting a novel coronavirus neutralizing antibody of non-diagnostic purpose. The new coronavirus neutralizing antibody detection method established by relying on HEK293hACE2 cells and fluorescein labeled new coronavirus spike glycoprotein (namely PE-S1)can quickly complete detection of the new coronavirus neutralizing antibody in serum within 12h, and has the characteristics of strong specificity, high sensitivity, good repeatability, simple operation, low laboratory requirements, high safety and the like.
Owner:ACROBIOSYSTEMS INC
Infectious bovine rhinotracheitis virus strain and preparation method and application thereof
ActiveCN102337250AEasy to operateHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCytopathic effectFluorescence
The invention discloses an infectious bovine rhinotracheitis virus strain and a preparation method and application thereof. A wild strain of infectious bovine rhinotracheitis virus is found. The preparation method comprises the following steps of: firstly, identifying a sample by a PCR (Polymerase Chain Reaction) and a fluorescence PCR; then adding bovine serum which is detected to be free of IBRV (Infectious Bovine Rhinotracheitis Virus) to MDBK (Madindarby Bovine Kidney) cells by means of a culture medium, cultivating for a certain period of time so as to grow single-layer cells; after inoculating the single-layer cells with the processed sample, observing the cytopathic effect; after taking a pathological cell sample and identifying the pathological cell to be positive by the PCR, measuring TCID50 (Tissue Culture Infective Dose) as 106.2, and carrying out the virus neutralization test, wherein the TCID is 27; then inoculating the sample into the single layer cells; and when 70 percent to 80 percent of cells undergo the cytopathic effect and storing the cells into a refrigerator at a temperature of -40 DEG C. The infectious bovine rhinotracheitis virus strain and the preparation method disclosed by the invention can be applied to preparation of an engineered vaccine for preventing the infectious bovine rhinotracheitis.
Owner:湖南普简生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com