Neutralizing single-domain antibody with function of resisting novel coronavirus SARS-Cov-2 and application of neutralizing single-domain antibody
A sars-cov-2, single-domain antibody technology, applied in antiviral agents, antiviral immunoglobulins, applications, etc., can solve the problems of limited and difficult changes in the structure of S protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
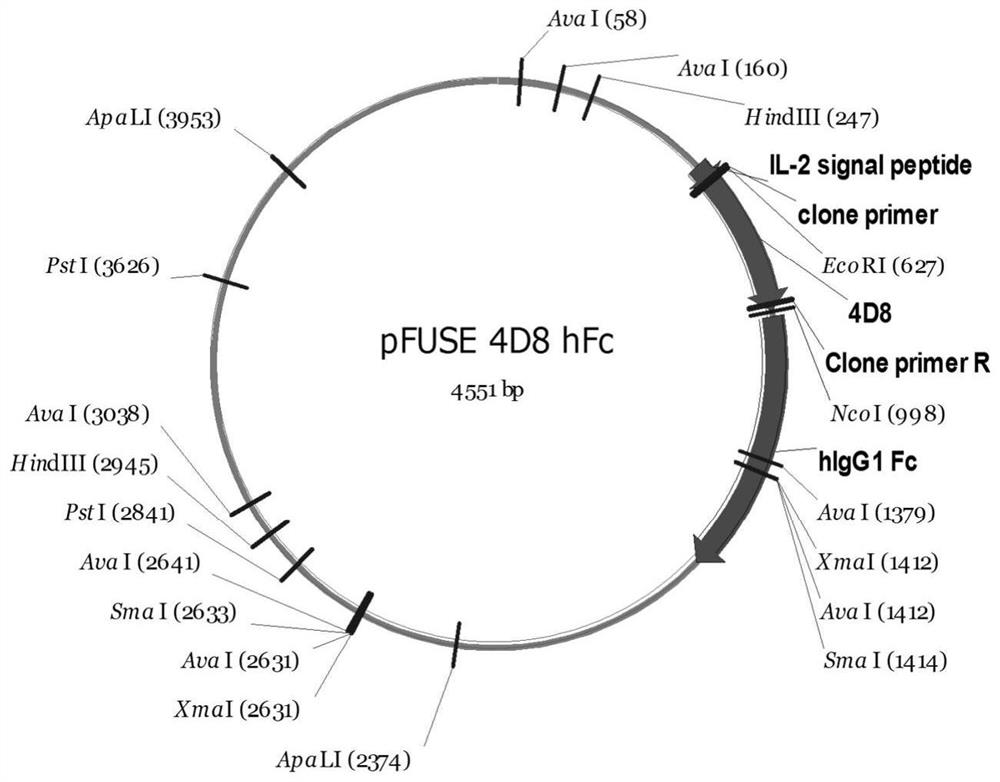
Image
Examples
Embodiment 1
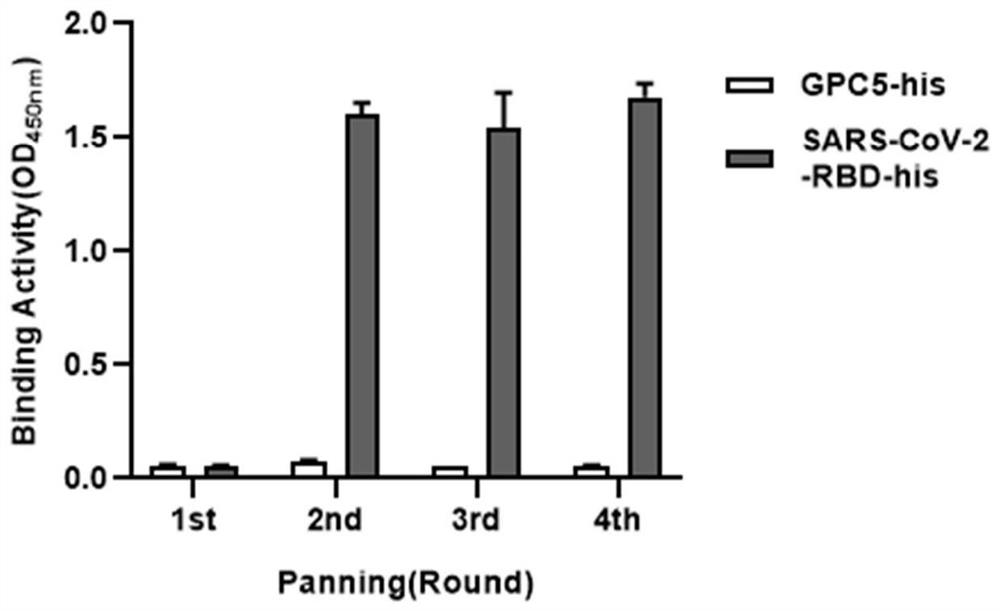
[0031] This example is to screen all human single domain antibodies targeting SARS-CoV-2-RBD.
[0032] Tomlinson I&J phage library (Genservice Ltd, Cambridge, UK, the library size is 1.47×10) 8 ) antibody sequence as a template, clone the antibody heavy chain sequence of the library, and construct a single-domain antibody library. Using phage display technology, SARS-CoV-2-RBD-HIS (ARG330-VAL524) protein as antigen was screened in single domain antibody library.
[0033] The specific process is as follows:
[0034] The ELISA plate was coated with 50μg / ml SARS-CoV-2-RBD his antigen at 4℃ overnight, 50ul / well; Using PBS solution (PBS STM) containing 5% skim milk powder and 0.1% tween-20 to block the enzyme-labeled plate at room temperature for 1 hour; Single domain antibody phage library with 10 12 Pfu was mixed with 10% skim milk powder PBS solution (PBSM) 1:1, then incubated at room temperature. After 2 hours, it was transferred to a sealed ELISA plate of SARS-CoV-2-RBD his antig...
Embodiment 2
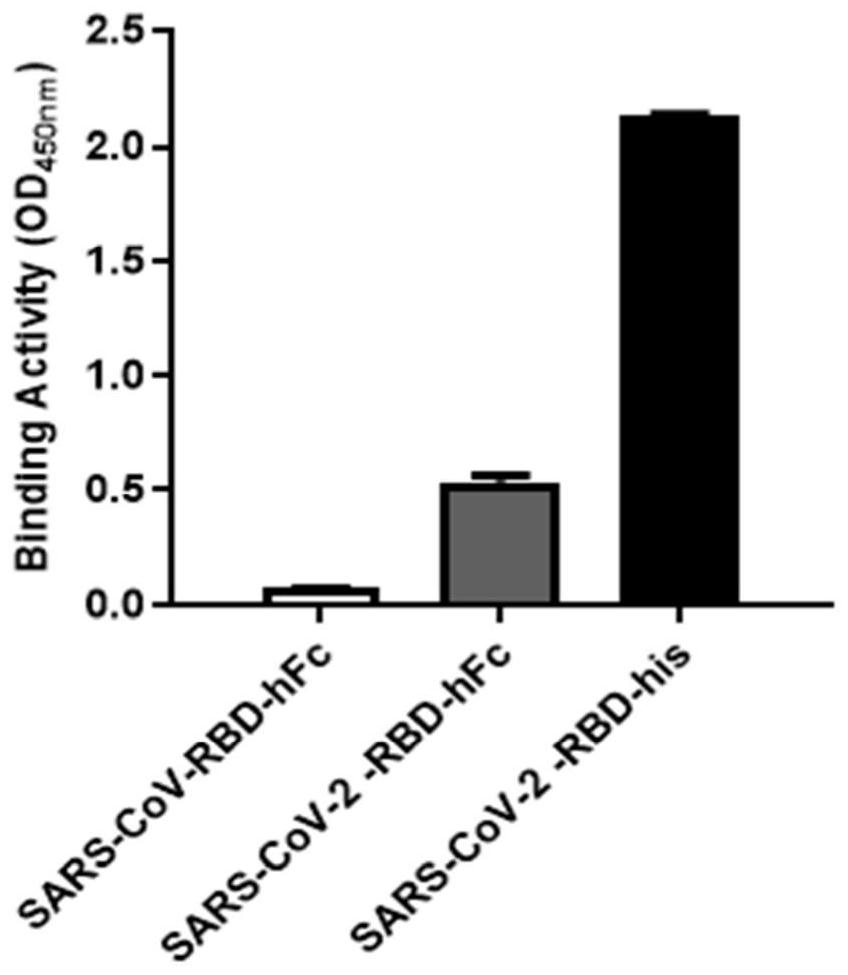
[0045] This example is the specific binding of phage 4D8 clone to SARS-CoV-2-RBD protein.
preparation Embodiment 1
[0046] The 4D8 phage of Example 1 was prepared, and the binding specificity of 4D8 clone with SARS-CoV-2-RBD protein was detected by Phage ELISA.
[0047] The specific process is:
[0048] The ELISA plate was coated with 5μg / ml of SARS-CoV-RBD-hFc, SARS-CoV-2-RBD-his and SARS-CoV-2-RBD-hFc at 4℃ overnight. Seal that enzyme labeled plate with 3% pbstm at room temperature for 1 hour; 4D8 phage was incubated with 6% PBSM at the ratio of 1:1 at room temperature for 2 hours, then added to the sealed enzyme-labeled plate (50μl / well), and incubated at room temperature for 1 hour; The enzyme-labeled plate was washed with 0.05%PBST for 3 times (340ul / well); The HRP / anti-M13 monoclonal conjugate was mixed with 5% pbstm at the ratio of 1: 4000, added into the washed enzyme-labeled plate (50μl / well), and incubated at room temperature for 1 hour; Wash the enzyme-labeled plate with 0.05%PBST for 5 times; Add TMB chromogenic solution into enzyme-labeled plate (100μl / well), develop color at r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com