CD47 nanobody and application thereof
A single-domain antibody, expression vector technology, applied in the field of biomedicine or biopharmaceuticals, can solve the problems of harsh storage conditions and low stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1: Expression and purification of human CD47 protein
[0133] (1) The nucleotide sequence of human CD47 was synthesized on the pCDNA3.1(-) vector, and then its extracellular segment sequence was subcloned into the pFUSE-IgG1 vector; (2) The Omega plasmid extraction kit was used to extract and construct pFUSE-IgG1-hCD47 (ECD) plasmid; (3) Culture HEK293F cells to an OD of 2.0×10 6 cells / mL; (4) Mix the plasmid and transfection reagent PEI 1:3 evenly, let it stand for 20min, and then add it to HEK293F cells at 37°C, 6% CO 2 Cultivate in a shaker incubator for 5-6 days; (5) collect the cell supernatant and combine with Protein A beads at room temperature for 1 hour; (6) wash the beads with phosphate buffer solution pH 7.0, and then use 0.1M pH 3.0 Glycine Elution protein; (7) ultrafilter the eluted protein into PBS, take a sample after measuring yield and carry out SDS-PAGE detection (detection result such as figure 1 shown), and the rest of the protein was store...
Embodiment 2
[0134] Example 2: Construction and screening of CD47 single domain antibody library
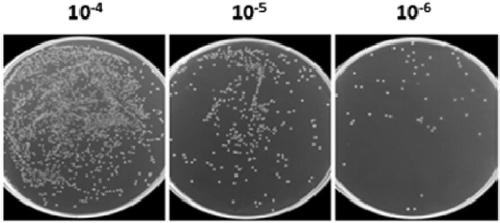
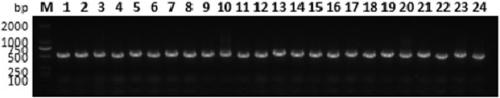
[0135] Library construction: Briefly, (1) mix 1 mg hCD47(ECD)-Fc antigen with Freund's adjuvant in equal volume, immunize a Xinjiang Bactrian camel once a week, immunize 7 times in total, and stimulate B cells to express antigen-specific (2) After 7 times of immunization, extract 100mL camel peripheral blood lymphocytes and extract total RNA; (3) Synthesize cDNA and amplify VHH by nested PCR; (4) Use restriction endonuclease 20 μg pMECS phage display vector (supplied by Biovector) and 10 μg VHH were digested with Pst I and Not I enzymes, and the two fragments were ligated; (5) The ligated product was transformed into electroporation-competent cells TG1 to construct a CD47 single-domain antibody library and measure the library capacity, Storage capacity is 2.5×10 9 CFU (results such as figure 2 shown). At the same time, 24 clones were randomly selected for colony PCR detection, and the res...
Embodiment 3
[0137] Example 3: Expression and purification of CD47 single domain antibody in eukaryotic cells HEK293 and detection of blocking function of single domain antibody by flow cytometry
[0138]Eukaryotic cell HEK293F expresses CD47Nb-Fc fusion protein: (1) Cloning the CD47Nb sequence with correct sequencing results into pFUSE-IgG4 vector (purchased from Invivogen), and extracting pFUSE-IgG4-Nb plasmid with Omega plasmid extraction kit; (2) ) to culture HEK293F cells to OD of 2.0×10 6 cells / mL; (3) Mix the plasmid and the transfection reagent PEI at a ratio of 1:3, let it stand for 20 minutes, and then add it to HEK293F cells at 37°C, 6% CO 2 Cultivate in a shaker incubator for 5-6 days; (4) collect the cell supernatant and combine with Protein A beads at room temperature for 1 hour; (5) wash the beads with phosphate buffer solution pH 7.0, and then use 0.1M pH 3.0 Glycine Elution protein; (6) the protein ultrafiltration of elution is entered in PBS, after measuring output, take...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com