Novel coronavirus SARS-Cov-2 resistant neutralizing single-domain antibody and application thereof
A sars-cov-2, single-domain antibody technology, used in antiviral agents, antiviral immunoglobulins, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
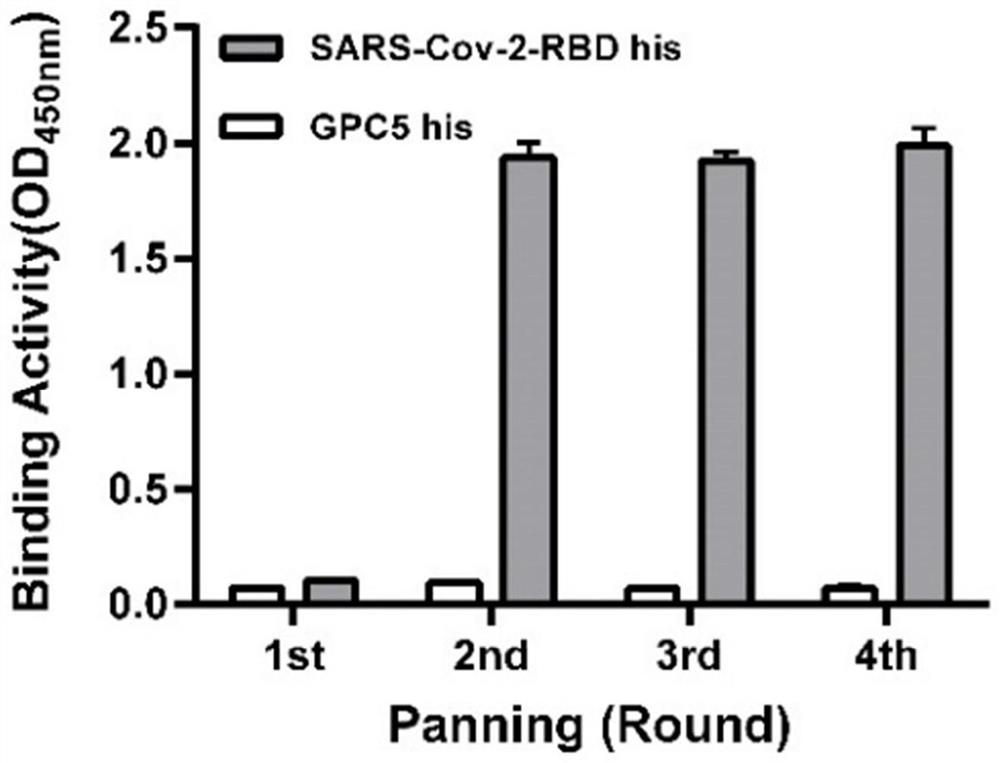
[0038] Example 1. Screening of fully human single domain antibodies targeting SARS-Cov-2-RBD
[0039] Using Tomlinson I&J phage library (Genservice Ltd., Cambridge, UK, the library size is 1.47×10 8 ) antibody sequence as a template, and the antibody heavy chain sequence of the library was amplified by PCR to construct a single domain antibody phage library.
[0040] Phage display technology was used to screen SARS-Cov-2-RBD his (Arg330-Val524) protein as positive antigen and SARS-Cov-2-RBD mut-hFC as negative antigen.
[0041] Use 50 μg / ml of the above-mentioned SARS-Cov-2-RBD his antigen and SARS-Cov-2-RBD mut-hFc to coat the immunoplate overnight at 4°C; use 5% skimmed milk powder, 0.1% Tween- 20 PBS solution at room temperature to block the immunoplate for 1 hour; the single domain antibody phage library obtained above was diluted with 10 12 Mix pfu with 10% skimmed milk powder PBS solution 1:1, incubate at room temperature for 2 hours, add the blocked SARS-Cov-2-RBD mut...
Embodiment 2
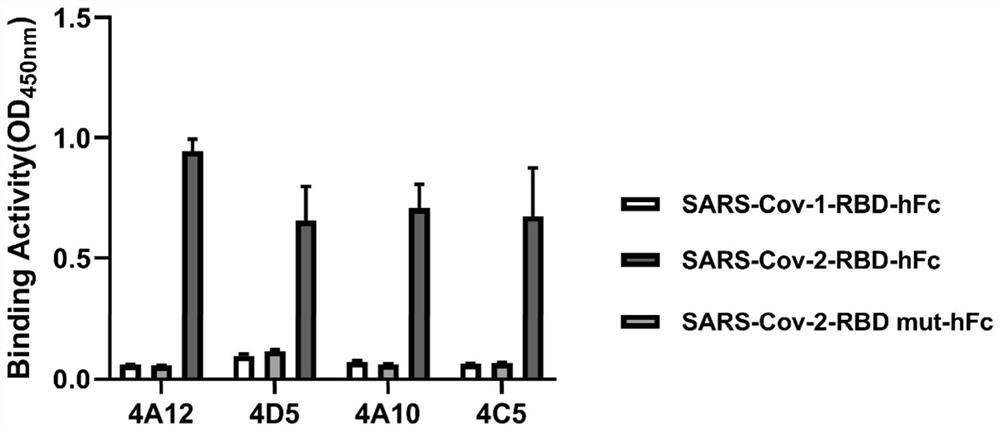
[0068] Example 2, Antigen specificity analysis of single domain antibody
[0069] In this example, ELISA was used to detect the binding status of the phages 4A12, 4D5, 4A10 and 4C5 in Example 1 to the SARS-Cov-2-RBD-hFc protein.
[0070] The specific process is as follows: use 5 μg / ml of SARS-Cov-1-RBD-hFc, SARS-Cov-2-RBD-hFc, and SARS-Cov-2-RBD mut-hFc to coat the immune plate overnight at 4°C; The PBS solution containing 3% skimmed milk powder and 0.05% Tween-20 blocked the immune plate at room temperature for 1 hour; respectively added 4A12, 4D5, 4A10, and 4C5 phages to each blocked immune plate (50 μl / well), and incubated at room temperature for 1 hour ;Wash the immunoplate 3 times with 0.05% Tween-20 in PBS solution (340ul / well); Mix HRP / Anti-M13 Monoclonal conjugate with 5% skimmed milk powder and 0.05% Tween-20 in PBS solution at a ratio of 1:4000 , added to the washed immune plate (50 μl / well), incubated at room temperature for 1 hour; washed the immune plate with 0.0...
Embodiment 3
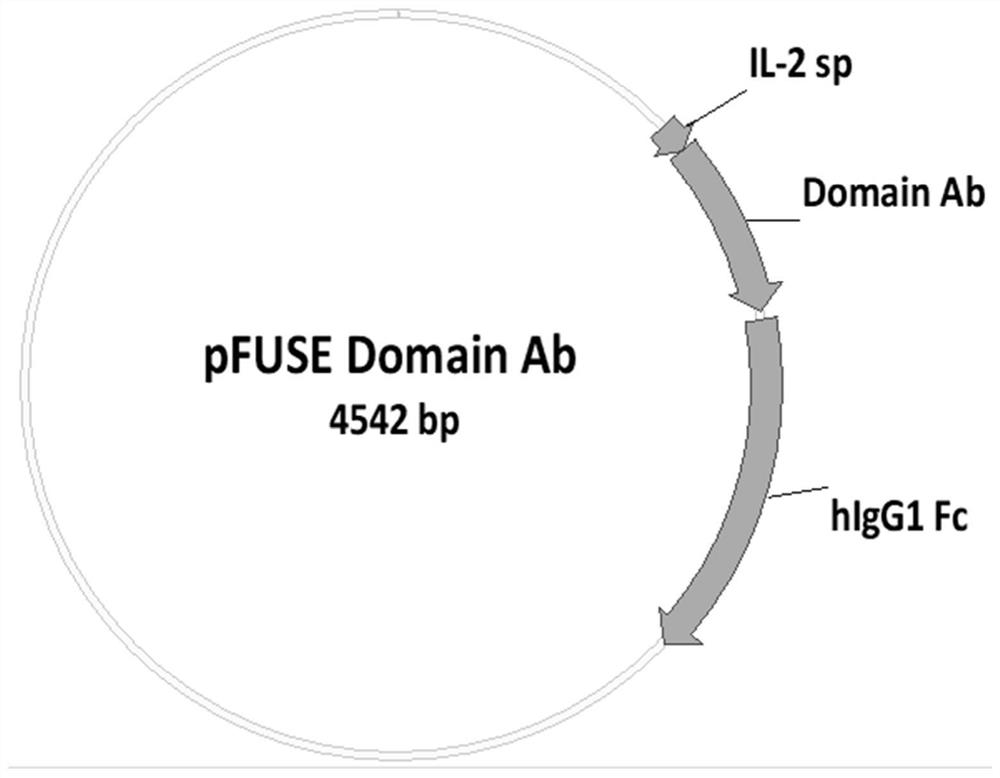
[0072] Example 3, Expression and Purification of Single Domain Antibody
[0073] Construct the eukaryotic cell expression vector pFUSE-4A12-hFC, pFUSE-4D5-hFC, pFUSE-4A10-hFC, pFUSE-4C5-hFC (Invivigen, San Diego, CA) of each antibody of Example 1, such as image 3 shown. After transfection in 293T cells, the supernatant was collected and purified with a protein A-Agarose separation column, and the purity of the antibody was detected by SDS-PAGE, as shown in Figure 4 shown.
[0074] The specific process is: Cloning the VH sequence of antibodies 4A12, 4D5, 4A10, and 4C5 into expression vectors pFUSE-4A12-hFc, pFUSE-4D5-hFc, pFUSE-4A10-hFc, pFUSE-4C5-hFc (Invivigen, San Diego, CA), plasmids were prepared. 5 million HEK293T cells were planted in a cell culture dish with DMEM medium supplemented with 10% fetal bovine serum, 100 U / ml penicillin, and 0.1 mg / ml streptomycin, and cultured in a 5% CO2, 37°C incubator. When the cell density reached 60-80%, 10 μg of pFUSE-4A12-hFc, p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com