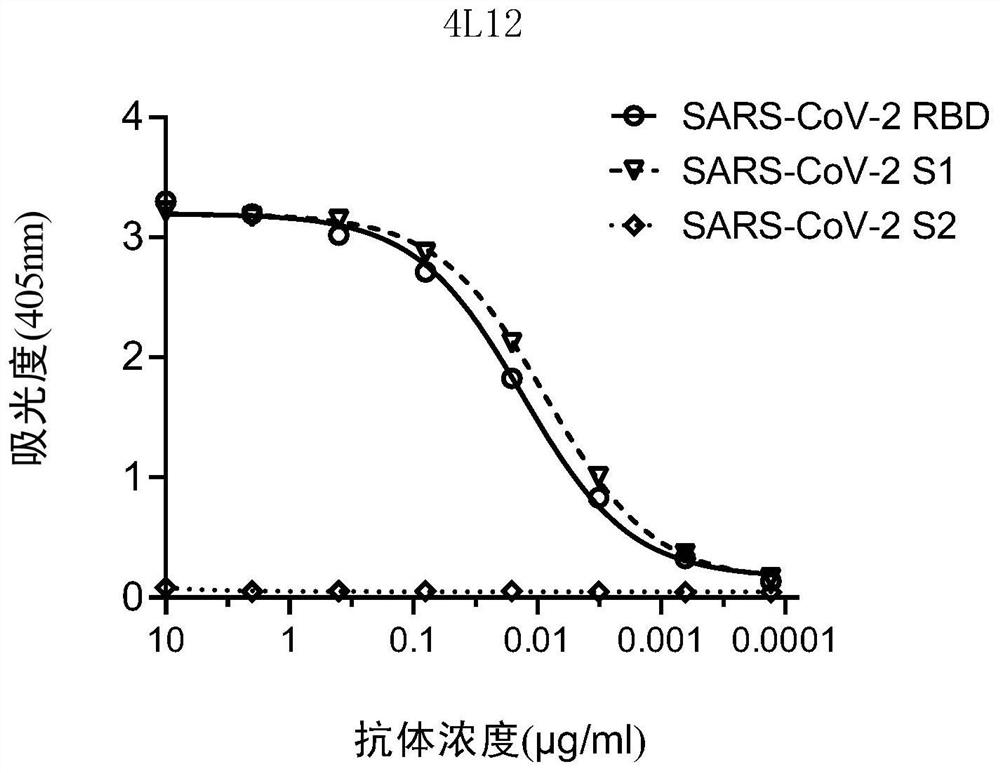
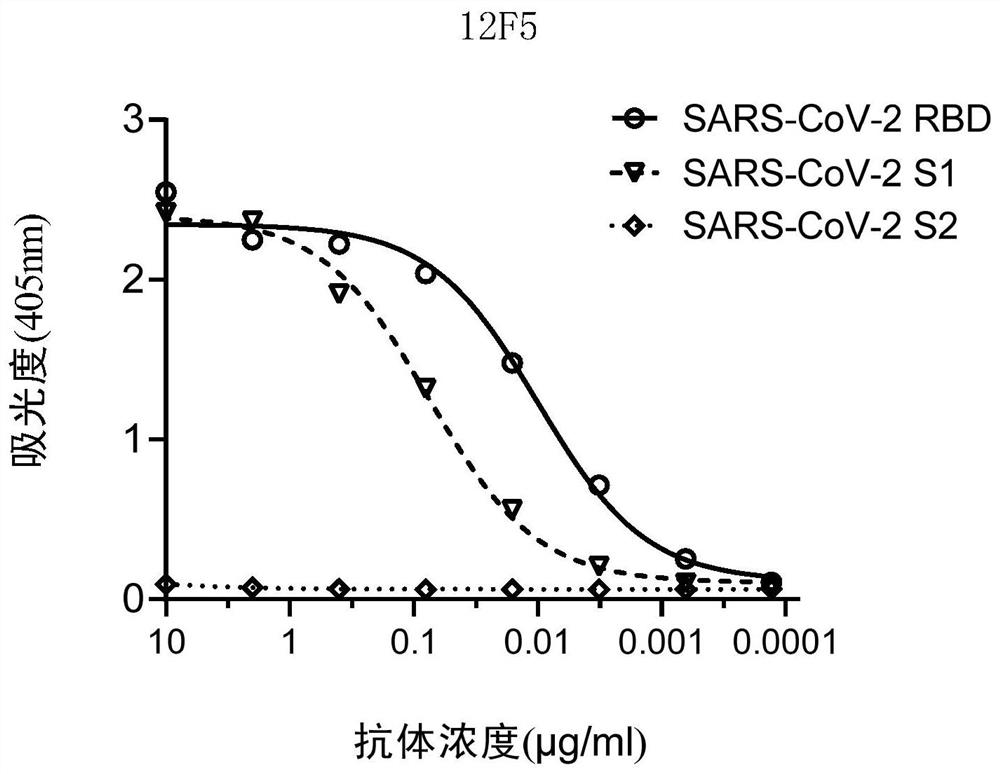
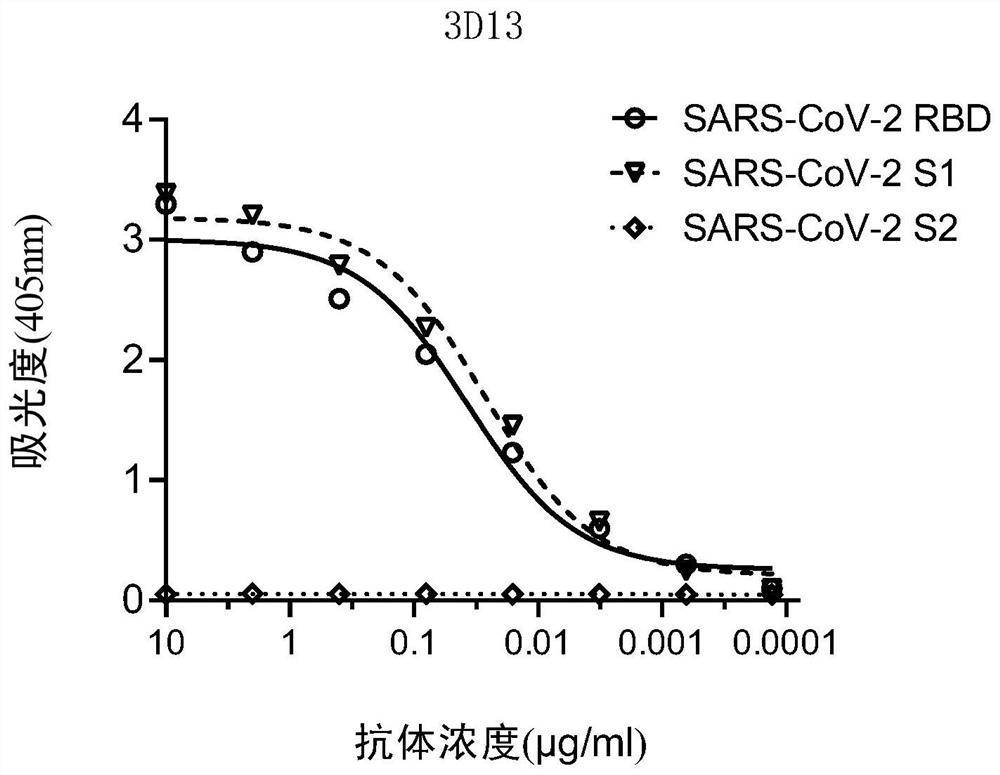
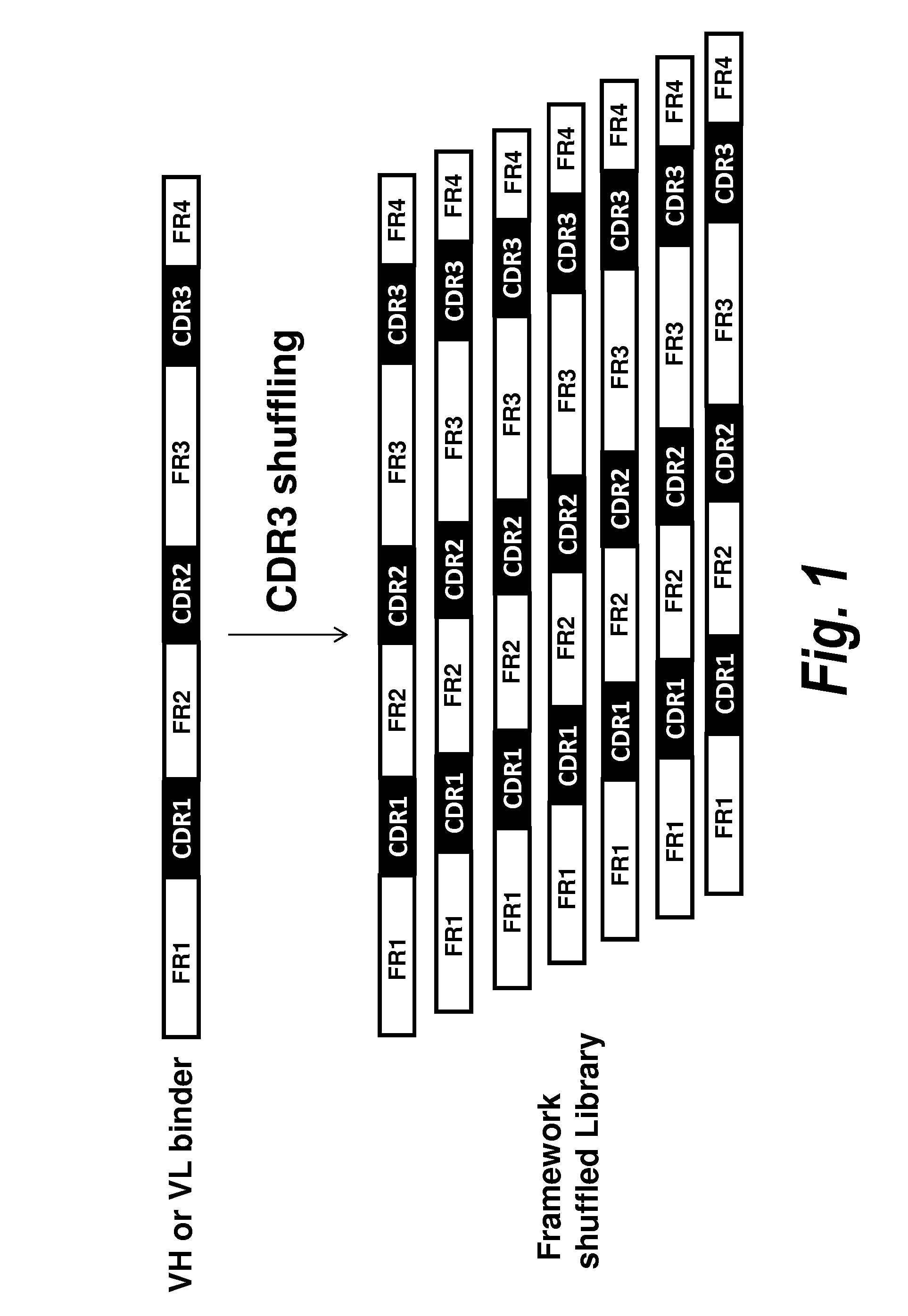
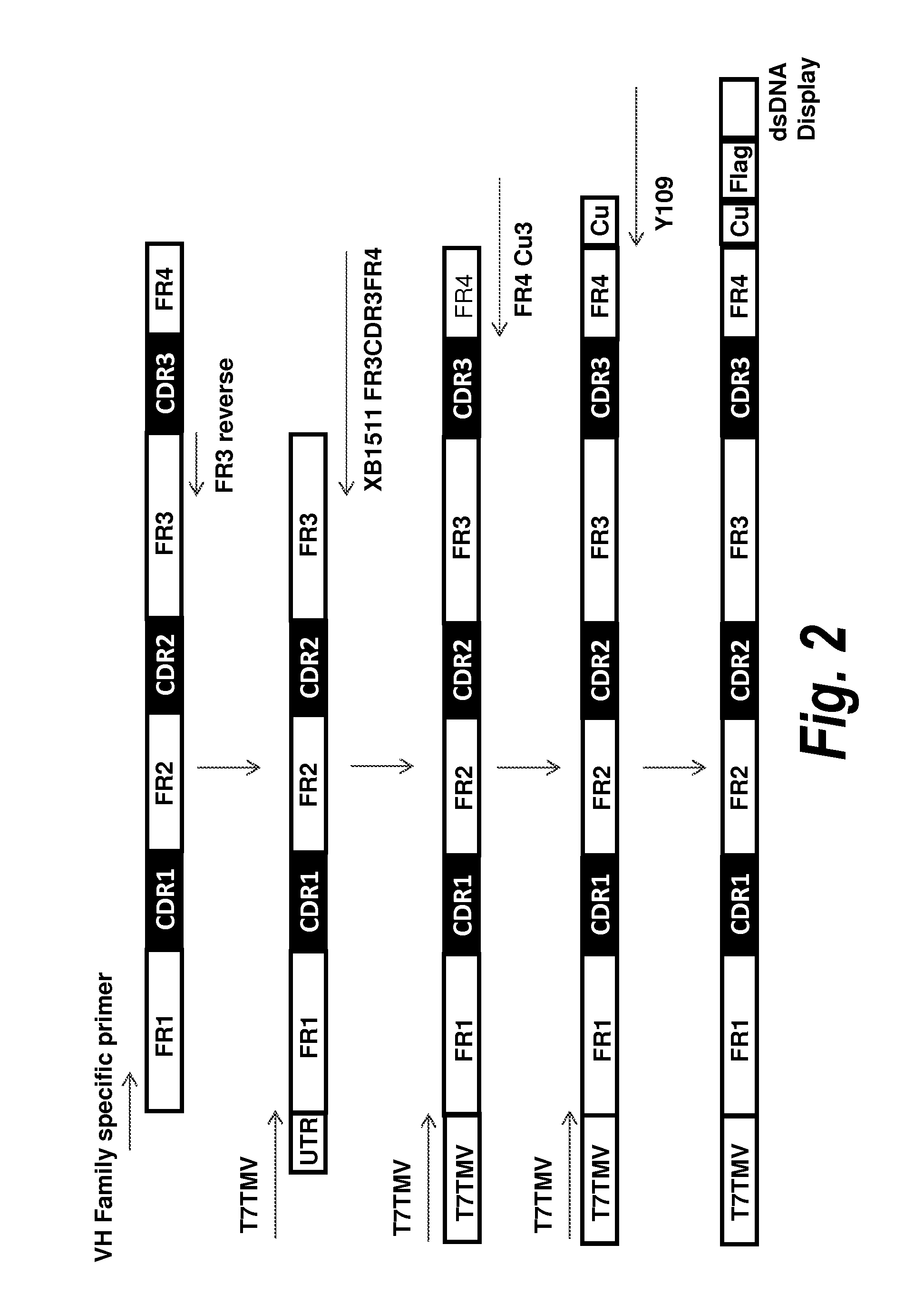
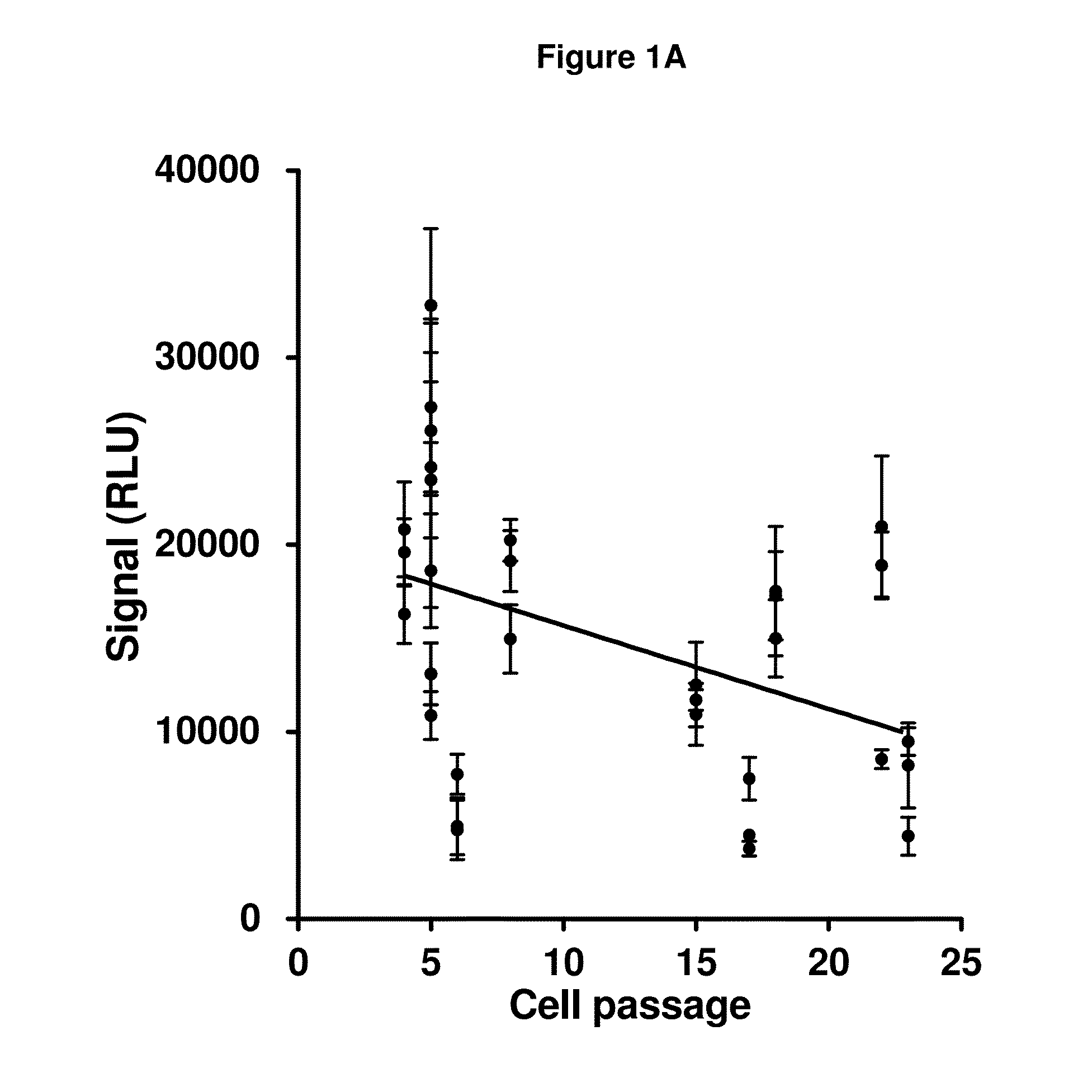
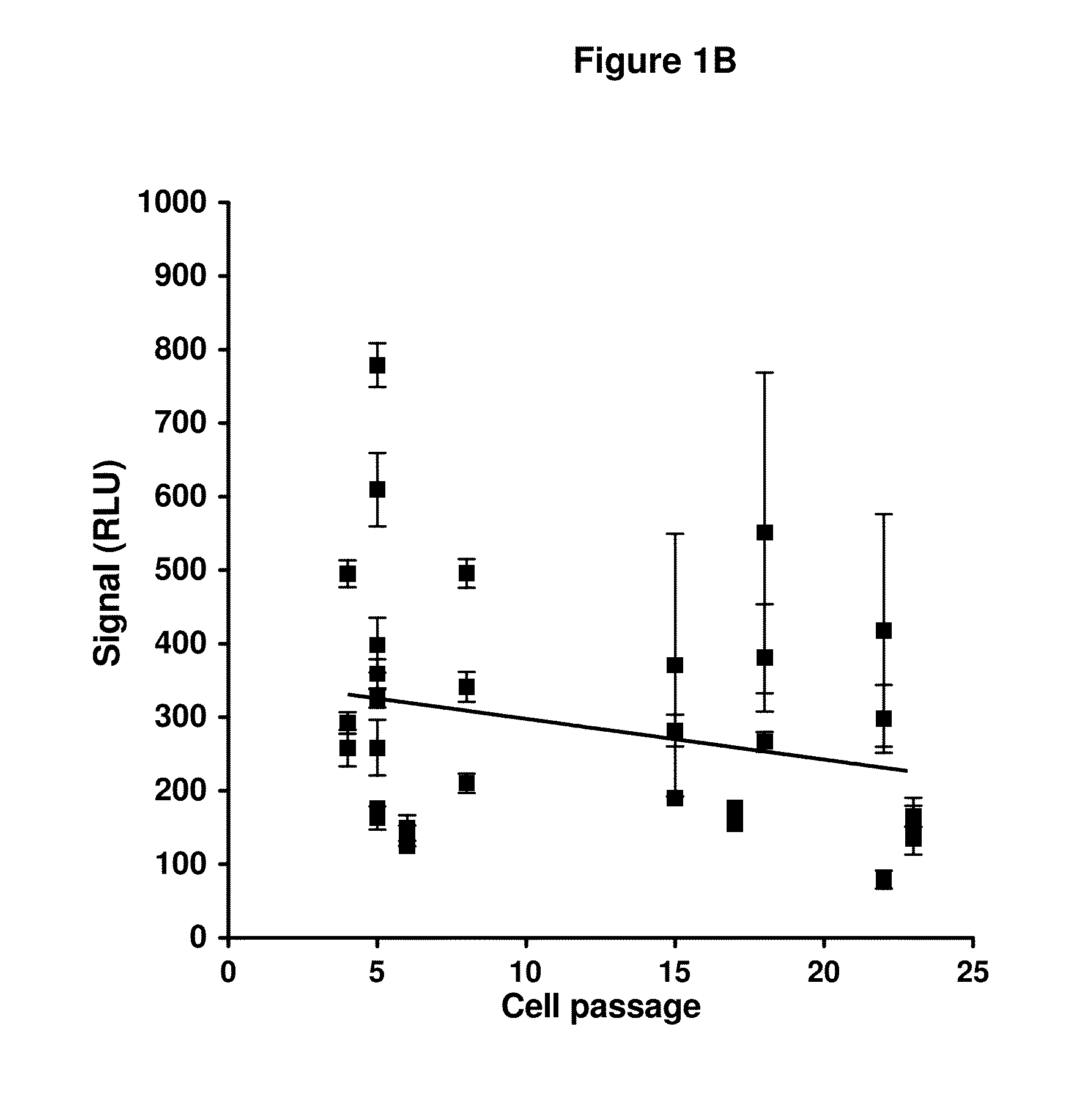
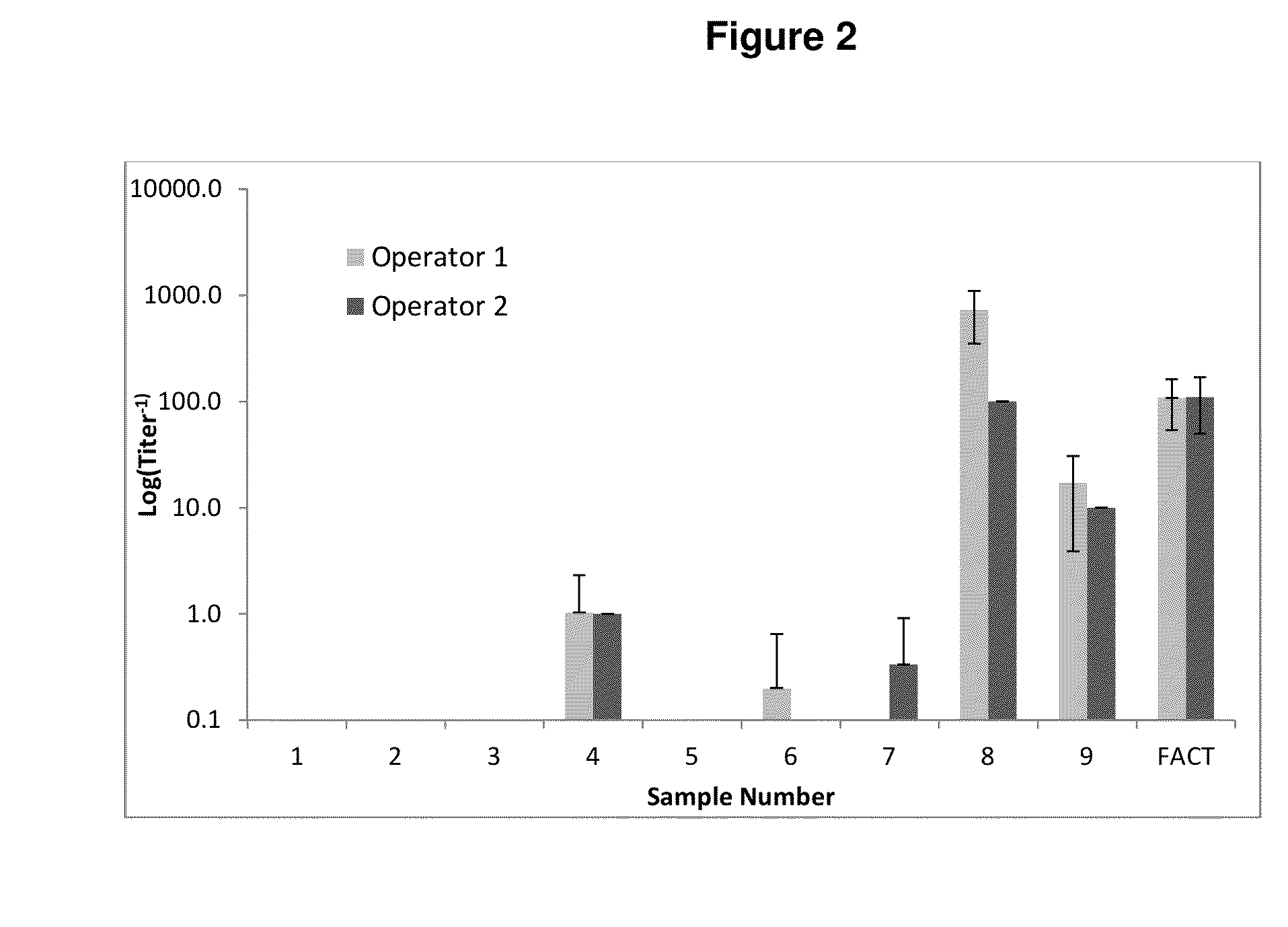
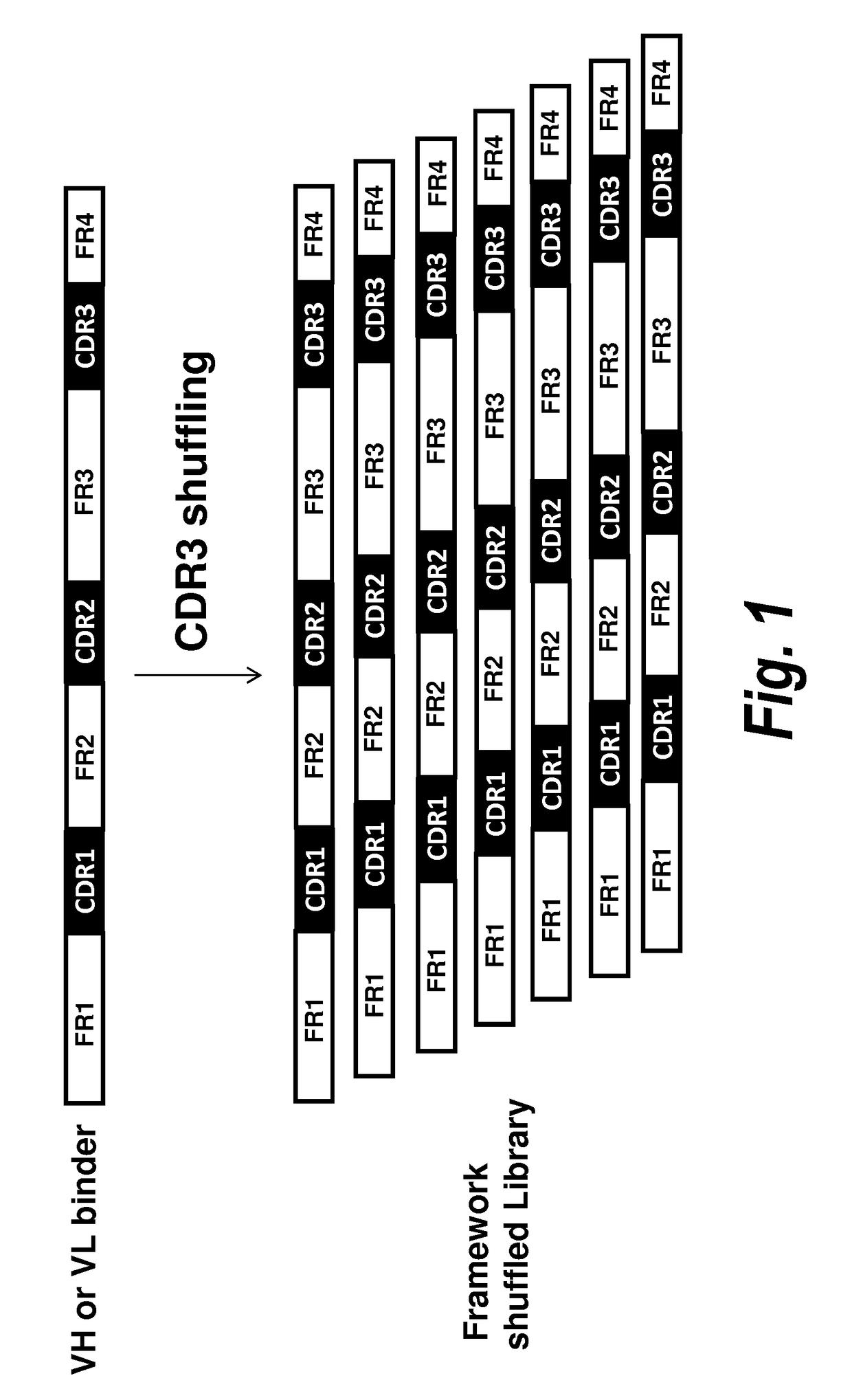
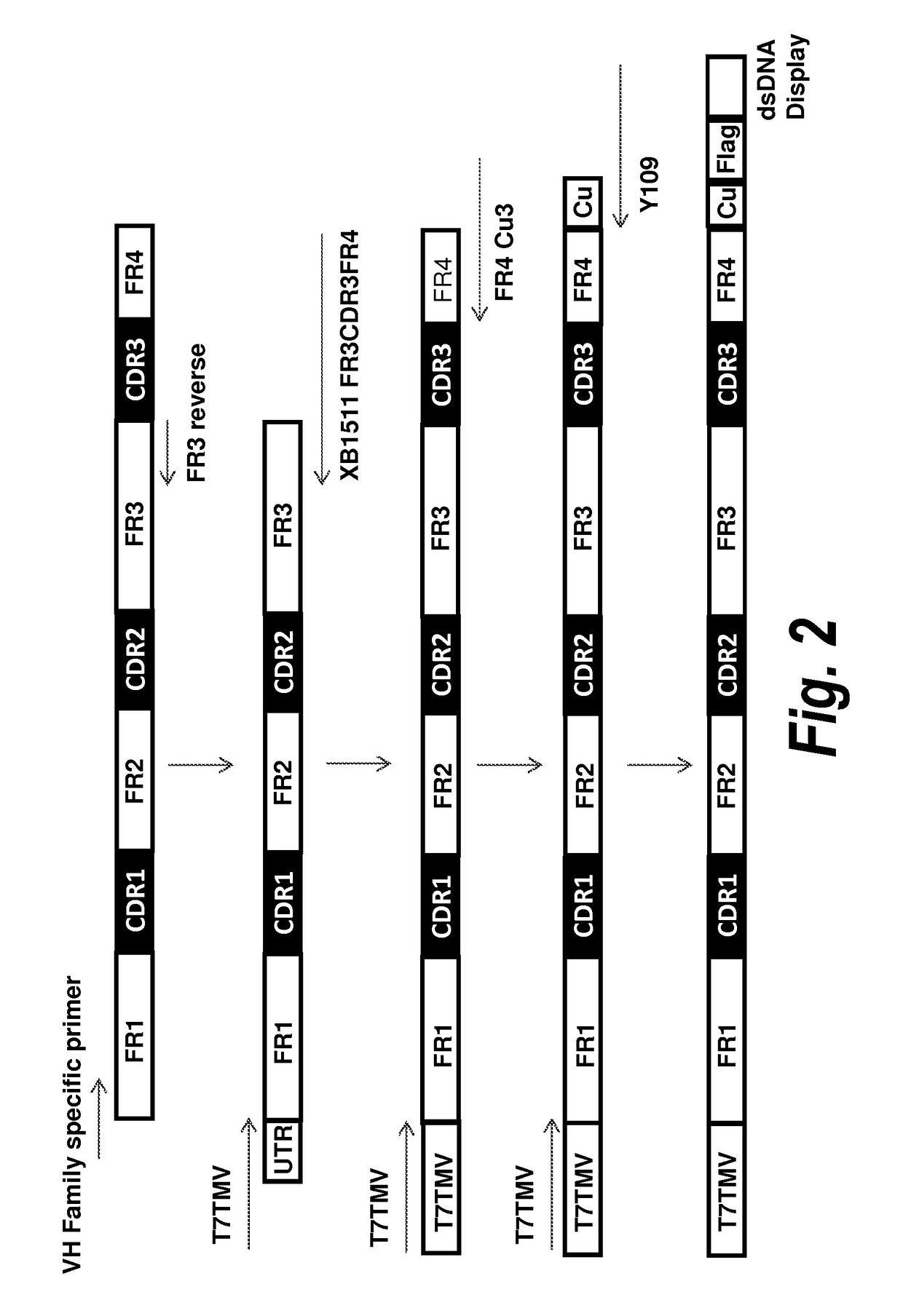
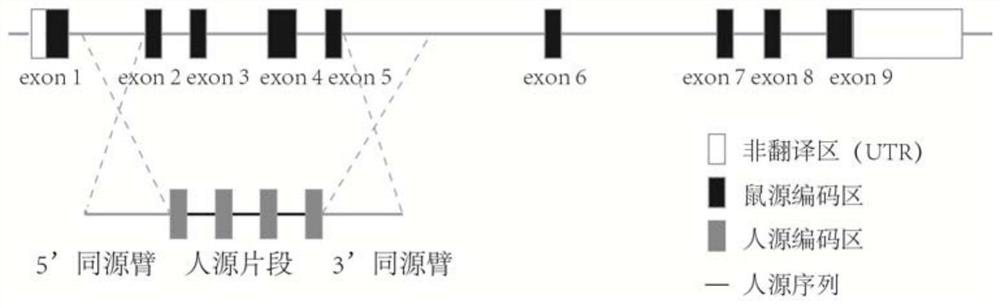
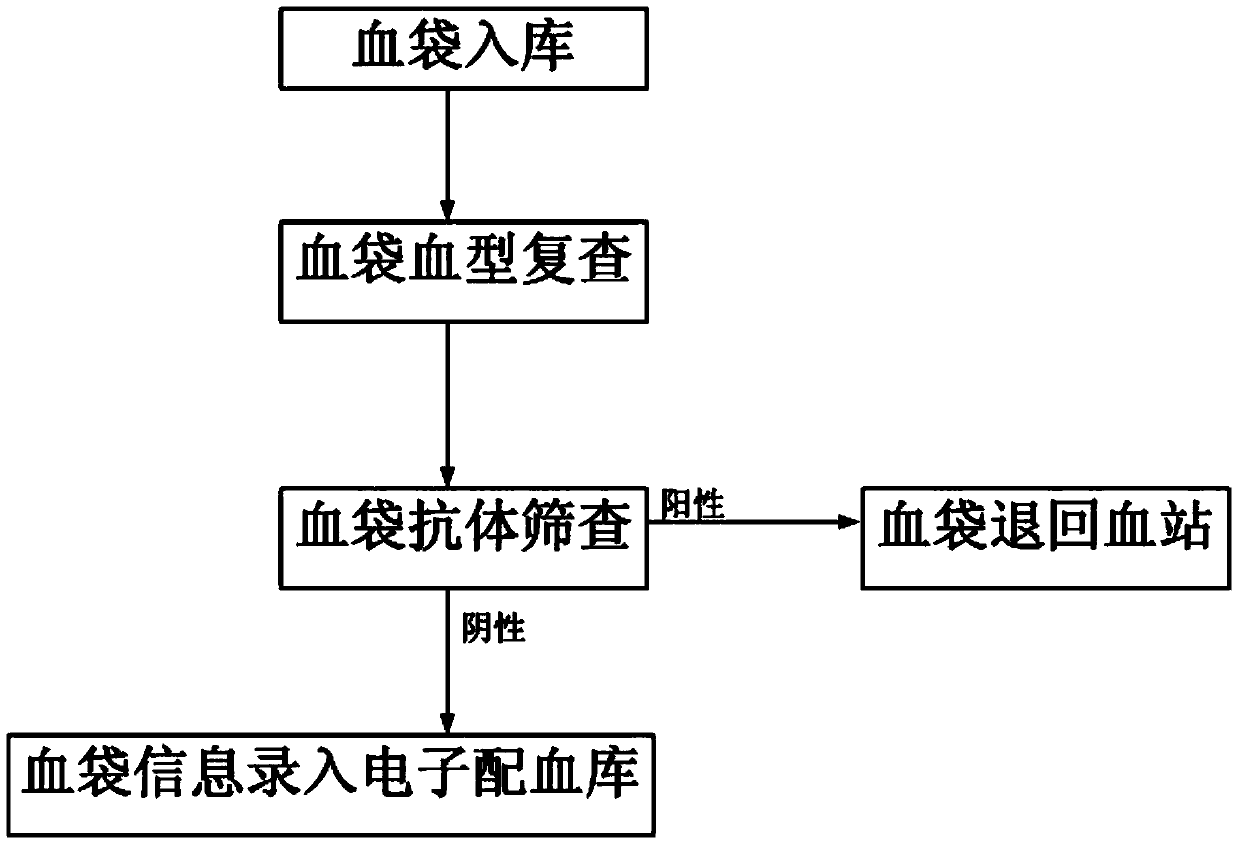
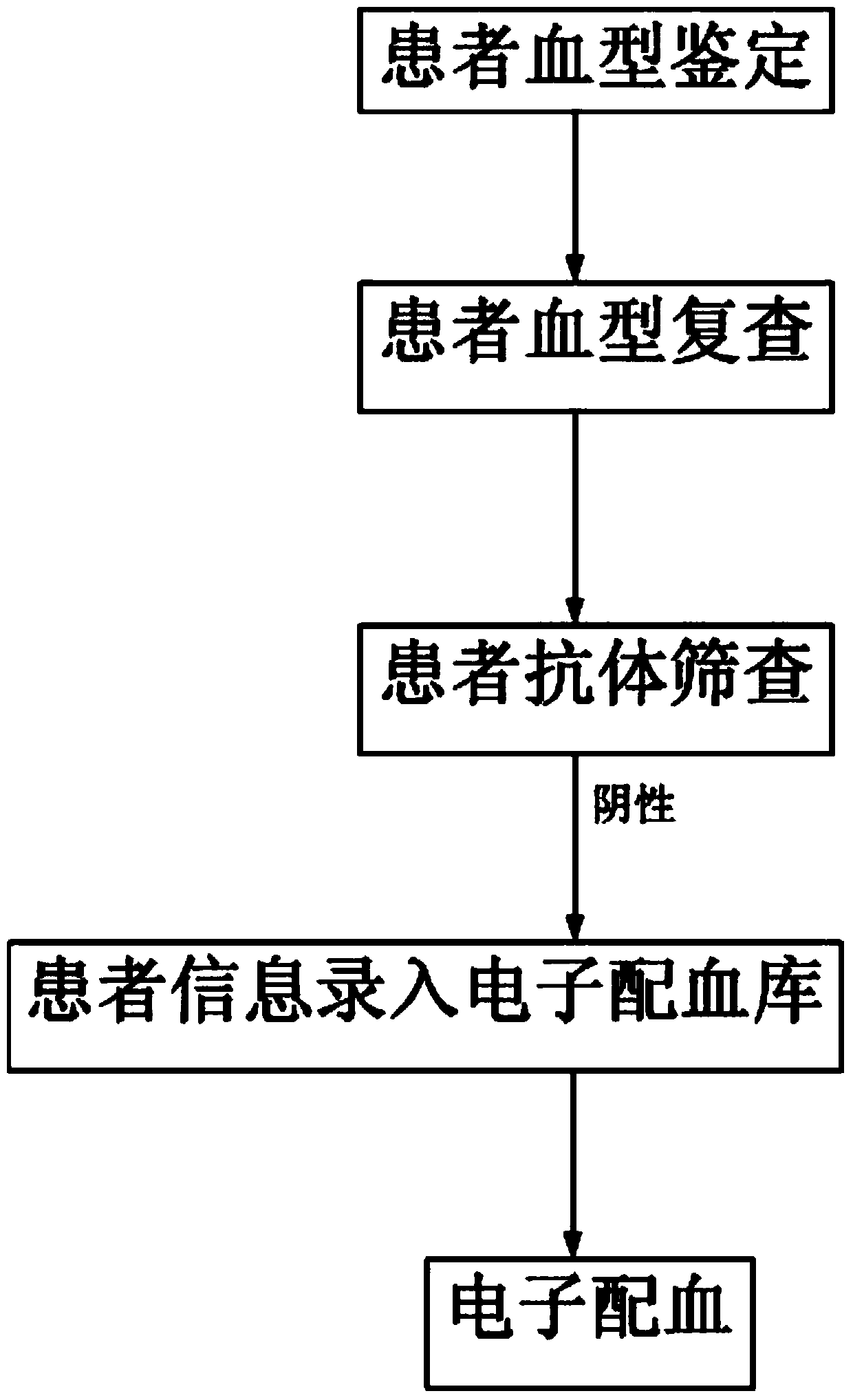
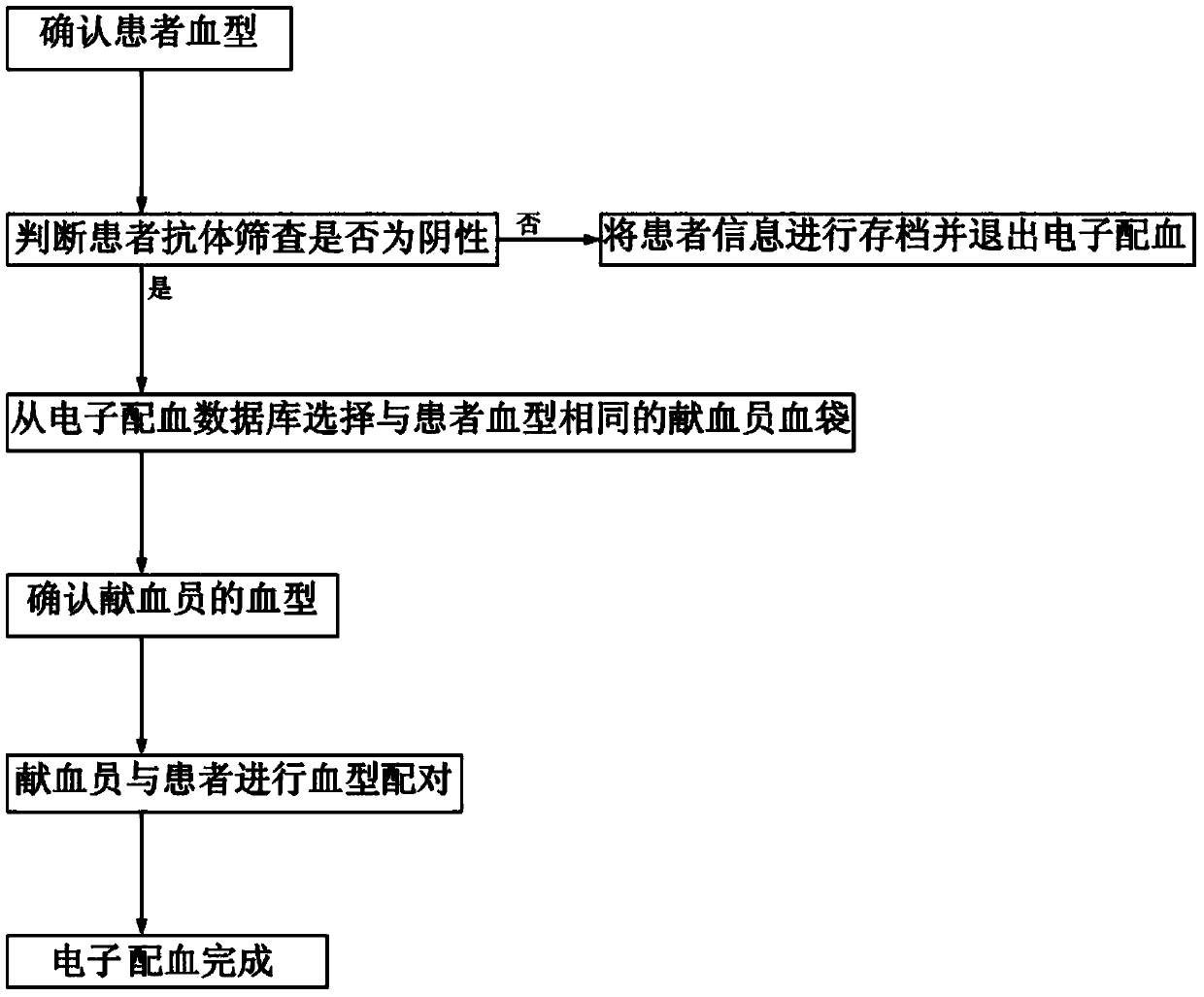
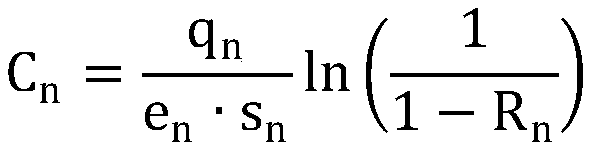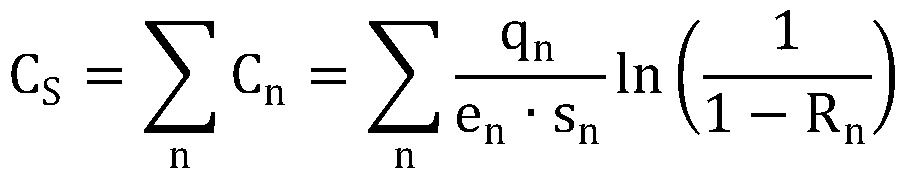Patents
Literature
104 results about "Antibody screening" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Small cell lung cancer associated antigens and uses therefor
InactiveUS7314721B2Tumor rejection antigen precursorsPeptide/protein ingredientsSerum igeADAMTS Proteins
Cancer associated antigens have been identified by autologous antibody screening of libraries of nucleic acids expressed in small cell lung cancer cells using antisera from cancer patients. The invention relates to nucleic acids and encoded polypeptides which are cancer associated antigens expressed in patients afflicted with small cell lung cancer. The invention provides, among other things, isolated nucleic acid molecules, expression vectors containing those molecules and host cells transfected with those molecules. The invention also provides isolated proteins and peptides, antibodies to those proteins and peptides and cytotoxic T lymphocytes which recognize the proteins and peptides. Fragments of the foregoing including functional fragments and variants also are provided. Kits containing the foregoing molecules additionally are provided. The molecules provided by the invention can be used in the diagnosis, monitoring, research, or treatment of conditions characterized by the expression of one or more cancer associated antigens.
Owner:NEW YORK HOSPITAL CORNELL MEDICAL CENT +2
Neutralizing antibody for resisting novel coronavirus SARS-Cov-2 and application thereof
ActiveCN111592595ABlock bindingGood effectImmunoglobulins against virusesAntiviralsNeutralizing antibodyPhage Display Techniques
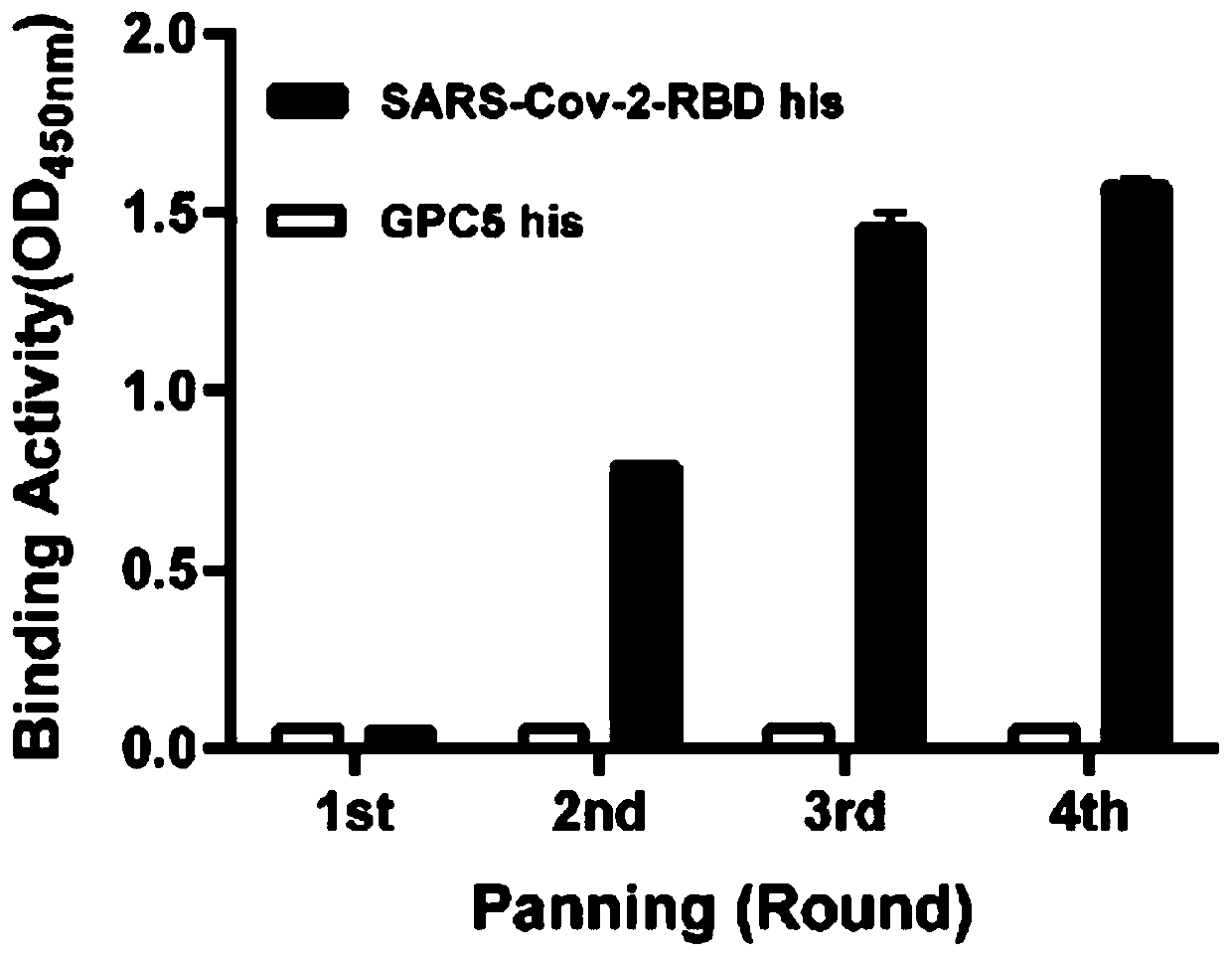
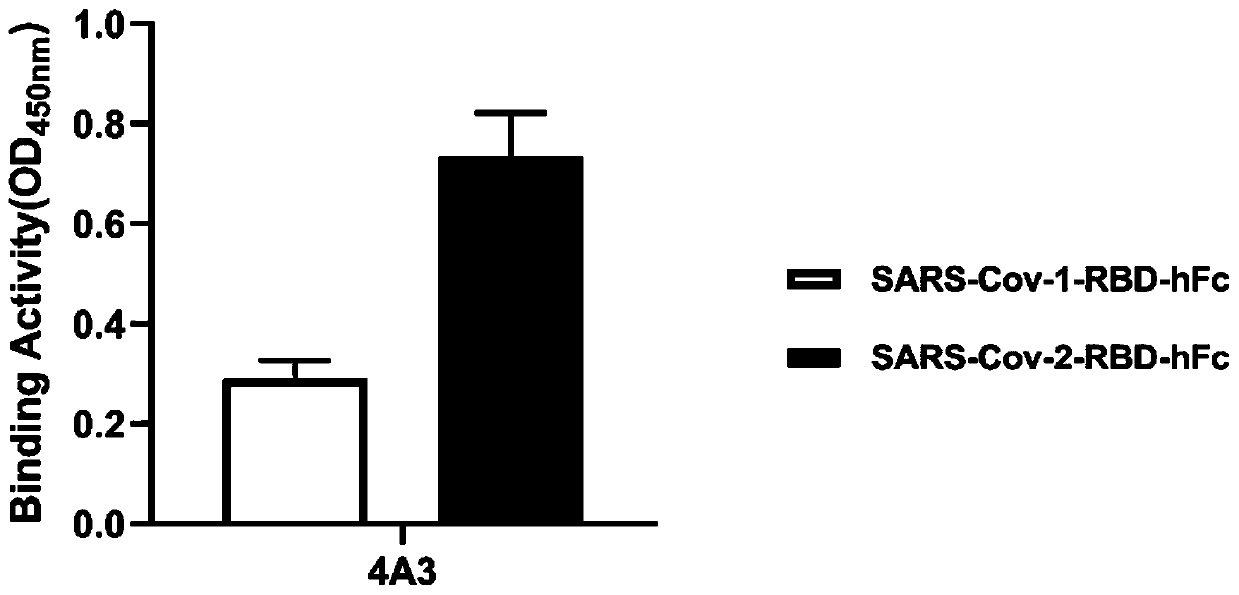
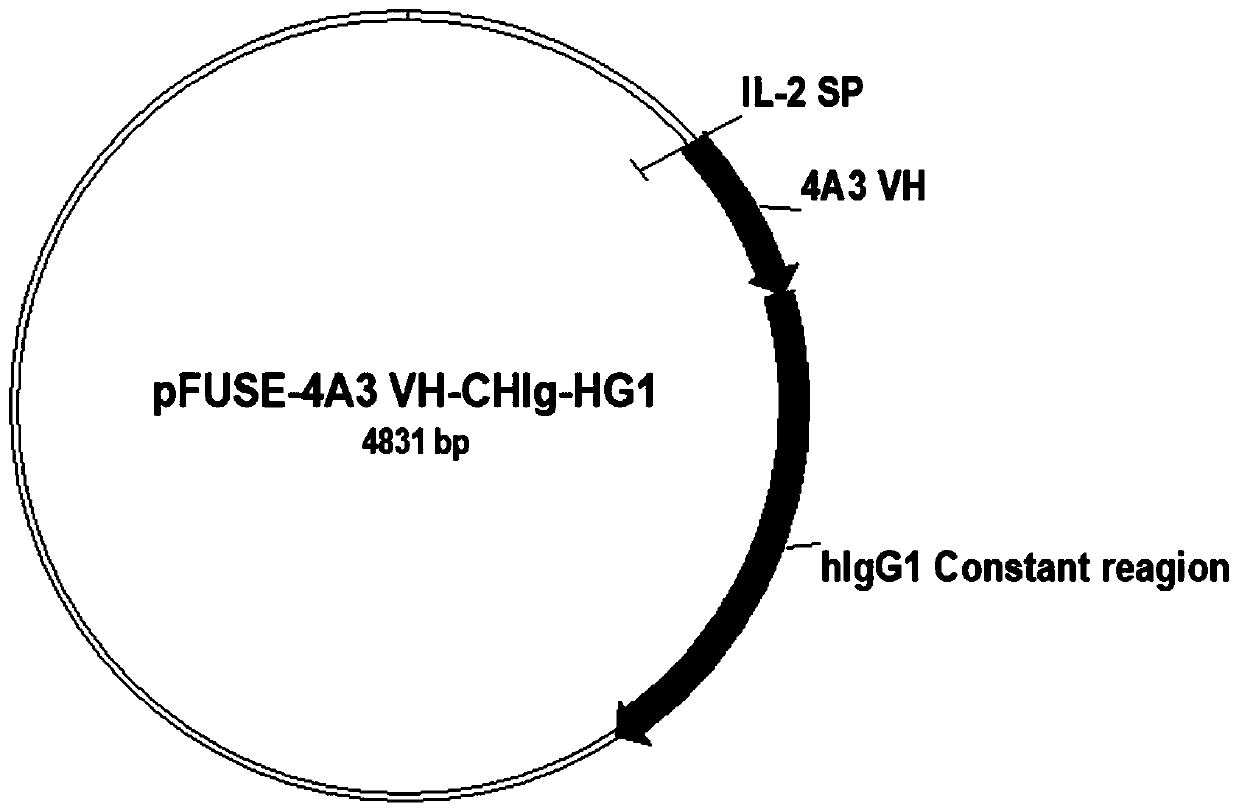
The invention relates to a neutralizing antibody for resisting a novel coronavirus SARS-Cov-2 and an application of the neutralizing antibody. The antibody at least comprises one of a heavy chain CDR1, a heavy chain CDR2, a heavy chain CDR3, a light chain CDR1, a light chain CDR2 and a light chain CDR3. The antibody can be used for preparing a diagnostic reagent or a diagnostic kit, a drug or a pharmaceutical composition for detecting, preventing and treating a COVID-19. According to the neutralizing antibody, differential antibody screening is carried out through a phage display technology ina manner of targeting SARS-Cov-2-RBD and SARS-Cov-1-RBD; the neutralizing antibody for resisting the novel coronavirus SARS-Cov-2 is obtained; binding of the SARS-Cov-2-RBD and ACE2 positive cells can be blocked; and the neutralizing antibody has a remarkable virus neutralizing effect on an SARS-Cov-2 pseudo virus and provides an effective alternative antibody drug for prevention and treatment ofthe COVID-19.
Owner:NANJING MEDICAL UNIV
Apparatus for forming and screening two-dimensional liquid droplet array, and use method thereof
The present invention discloses an apparatus for forming and screening a large-scale single-layer two-dimensional liquid droplet array, and a use method thereof. The apparatus comprises a micro-column array chip, a scraping plate for auxiliary spreading of a liquid droplet, a probe for controlling the liquid droplet, a three-dimensional translation table and other components, wherein a micro groove unit for trapping the single liquid droplet is formed by using the gap between micro-columns, and the liquid droplet is spread in the micro groove unit in a single-layer and single manner through gravity effect and capillary effect or through an auxiliary means so as to form the single-layer liquid droplet array. With the apparatus of the present invention, the accurate positioning and detection on the liquid droplet, the sucking on the target liquid droplet and the subsequent treatments can be performed. According to the present invention, the apparatus has characteristics of simple structure and easy operation; and the method has advantages of fast liquid droplet spreading, high flux, high liquid droplet trapping efficiency, liquid droplet position fixing and the like, and is suitable for single cell analysis and screening, single molecule analysis and screening, high-throughput gene screening, protein directed evolution, antibody screening, microbial research, drug screening, and other fields.
Owner:ZHEJIANG UNIV
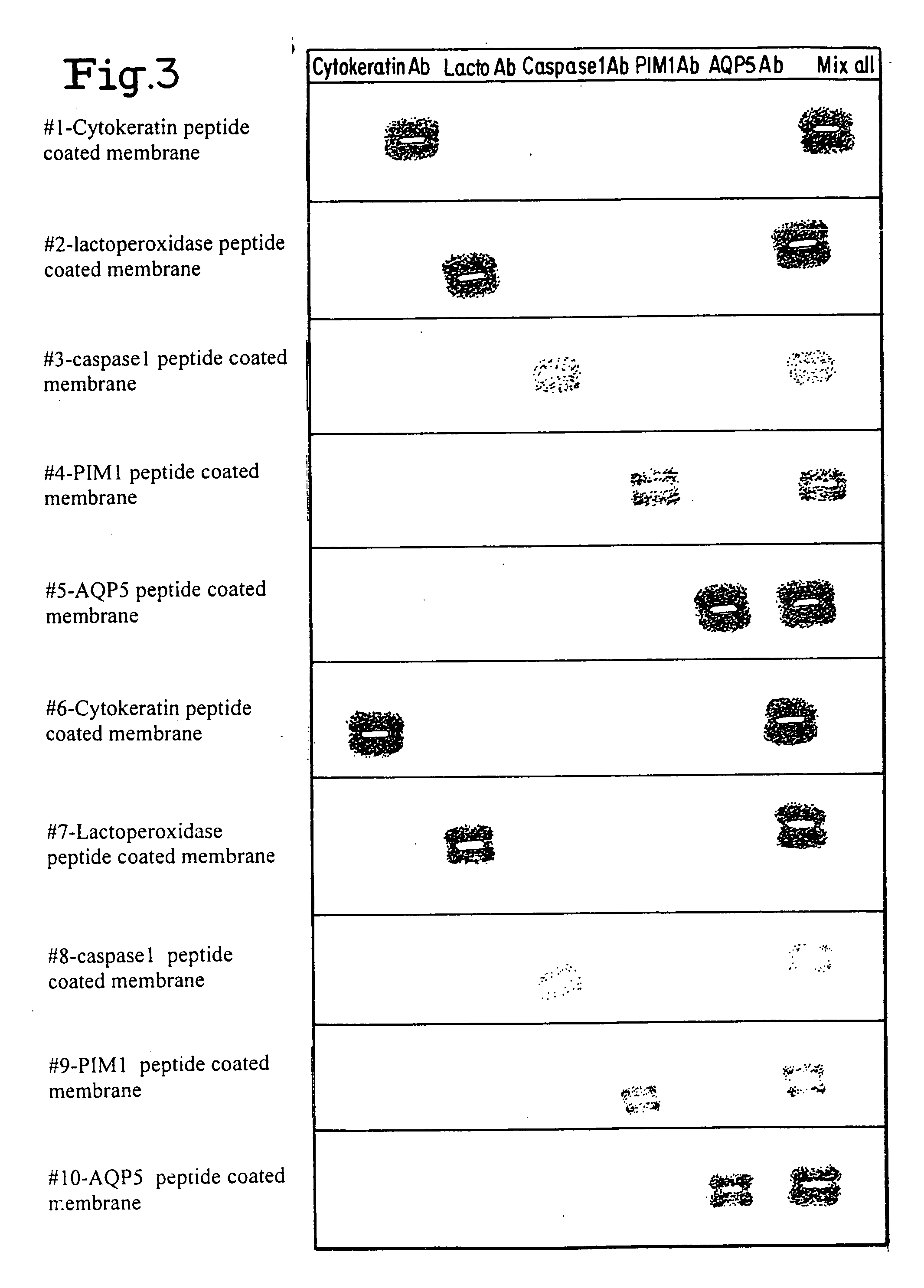
Layered peptide/antigen arrays - for high-throughput antibody screening of clinical samples
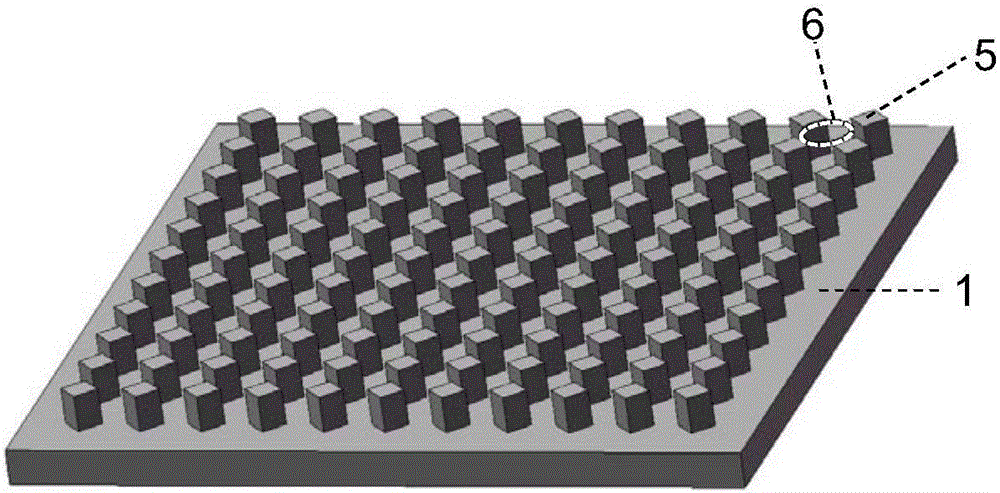
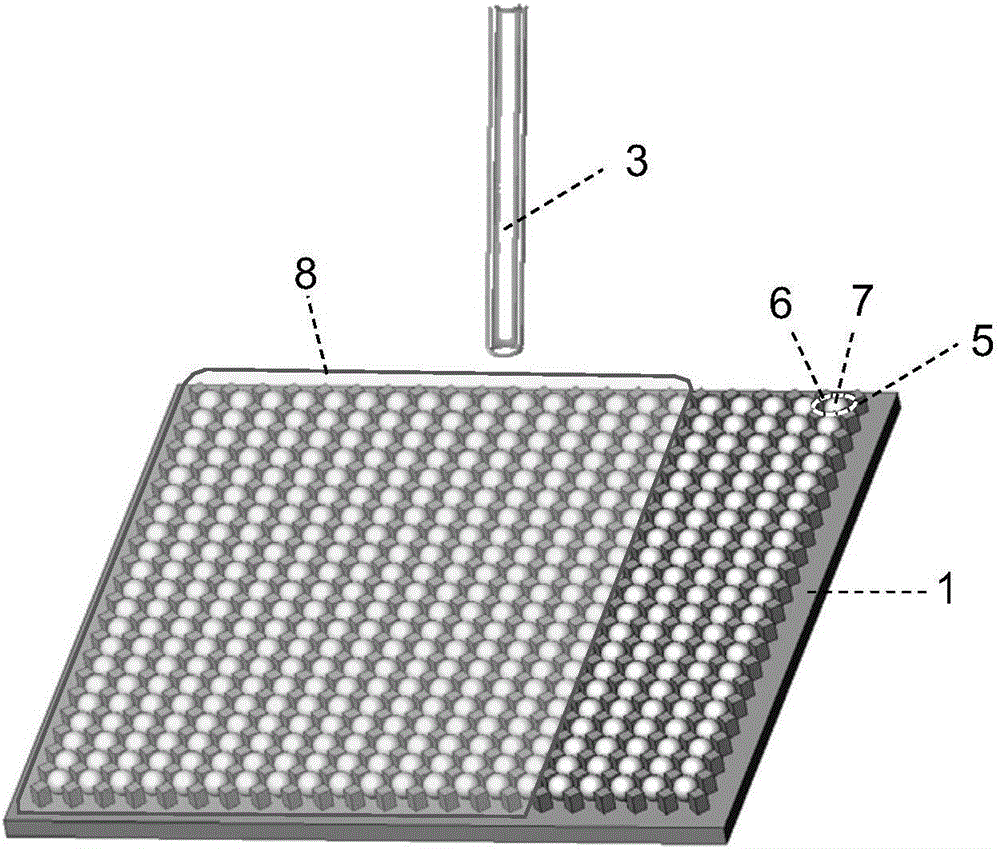
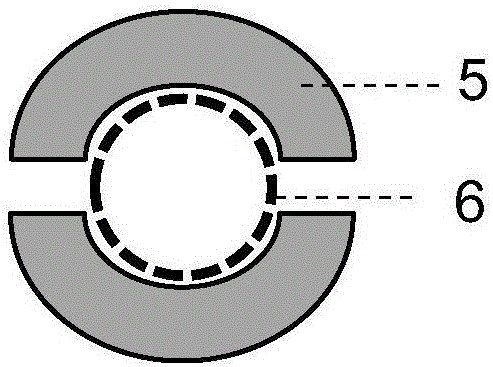
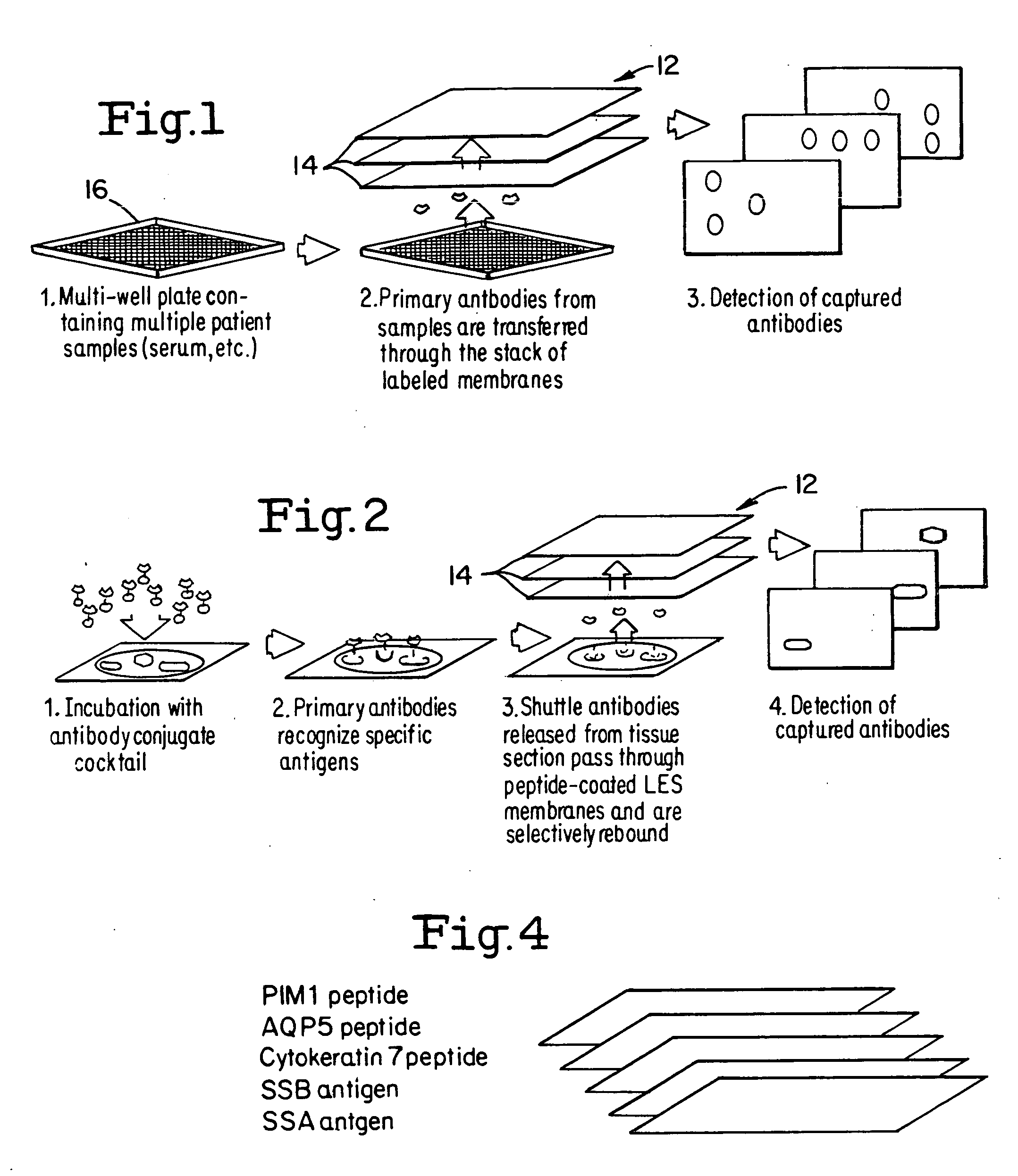
InactiveUS20060275851A1Quick and inexpensiveHigh-throughput detectionBioreactor/fermenter combinationsBiological substance pretreatmentsAntibody screeningMembrane configuration
A method and composition for the identification of biomolecule in a sample are disclosed. The method comprises obtaining a coated capture membrane stack comprising a plurality of capture membranes with each capture membrane coated with a different peptide. The membrane stack is exposed to a sample, and, after a given amount of time for the sample to permeate the membrane stack, the membrane stack is removed from the sample carrier and the capture membrane to which the biomolecule adheres is identified.
Owner:EMMERT BUCK MICHAEL R +1
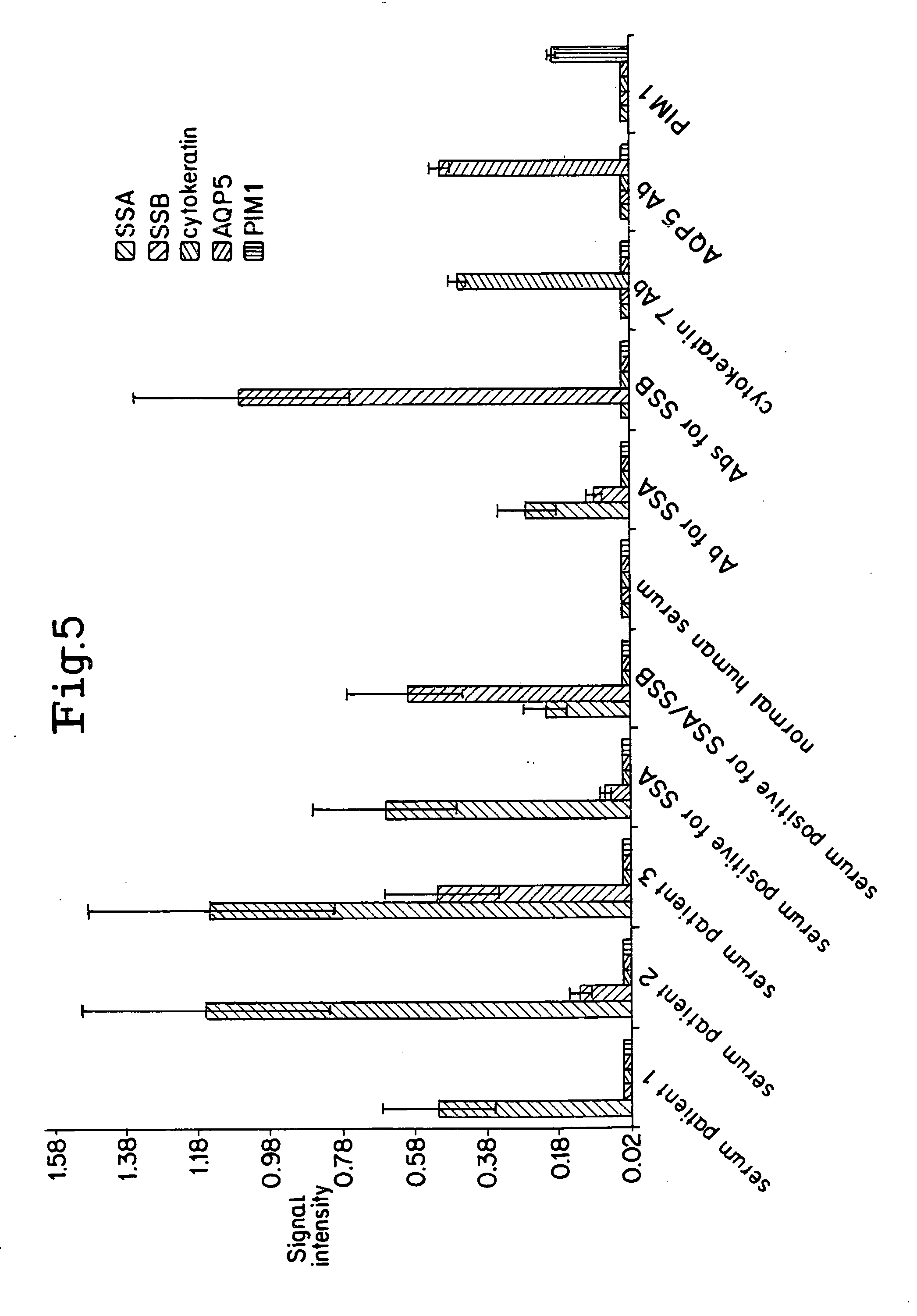
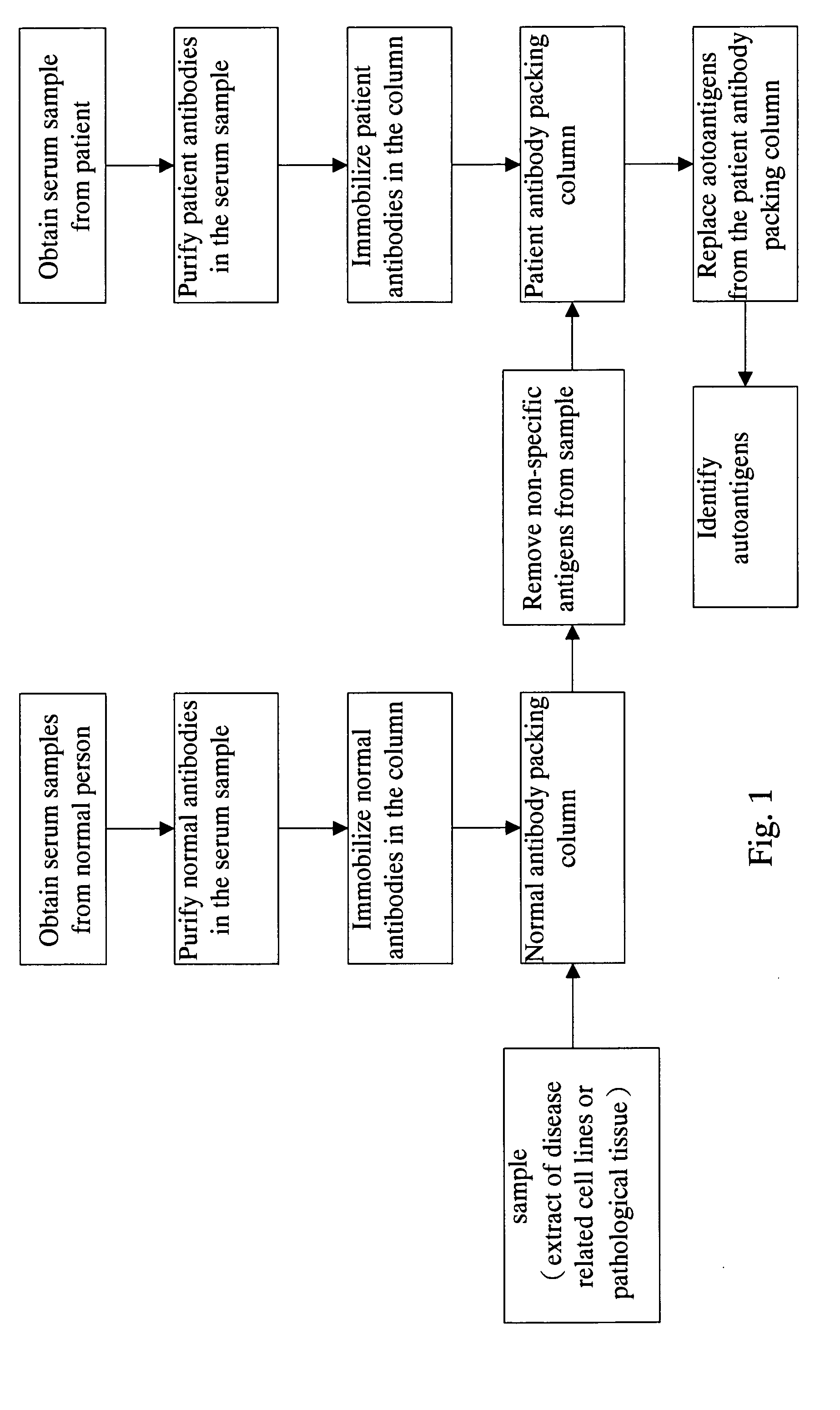
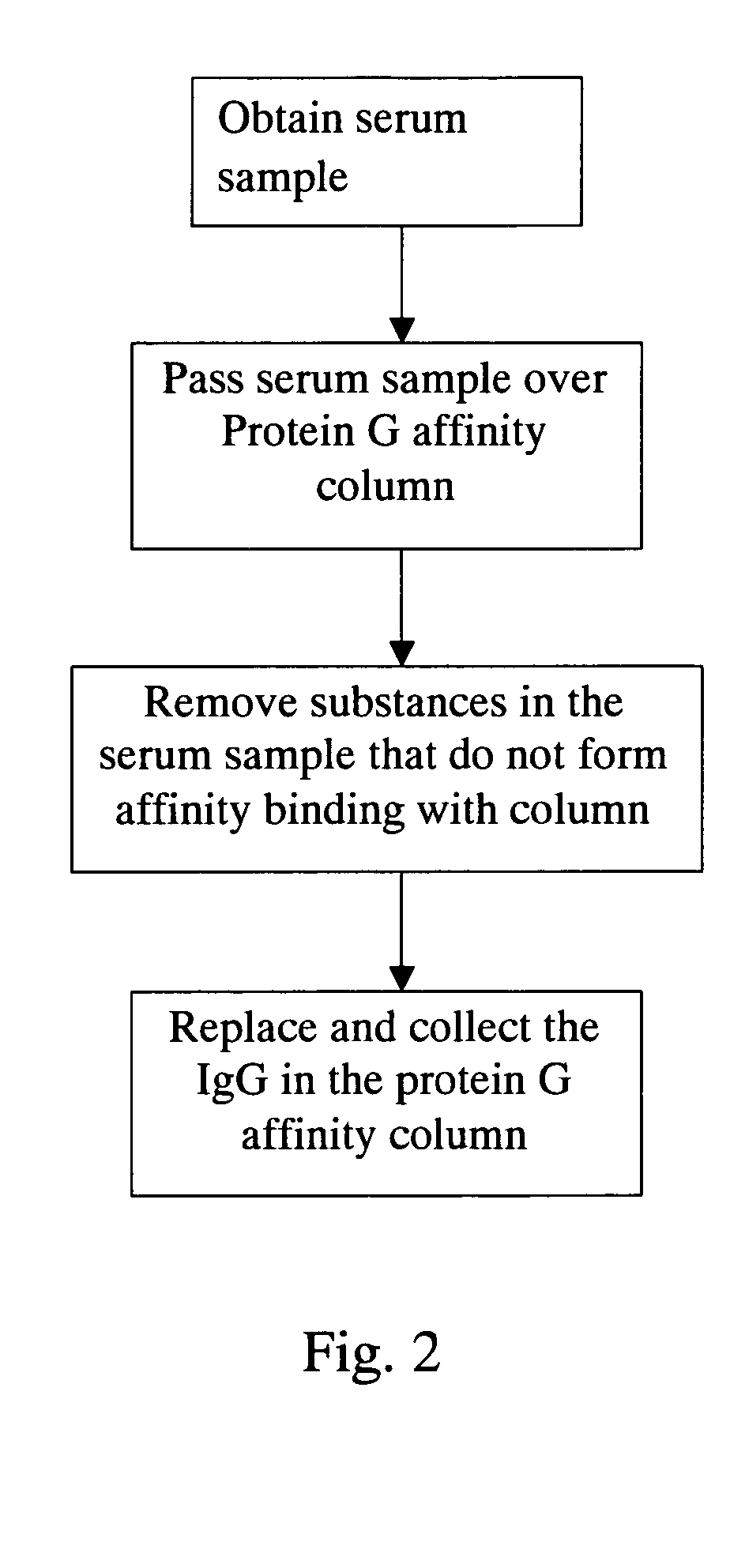
Method for screening autoantigen
InactiveUS20050124076A1Increase opportunitiesEasy to identifyComponent separationOther chemical processesScreening methodMass Spectrometry-Mass Spectrometry
The present invention relate to an autoantigen screening method by combining immunoaffinity chromatography, disease-related cell lines, and mass spectrometry technology, which offers greater screening efficiency and speed than known technologies. The method for screening autoantigen by using autoantibodies in patient's body by utilizing the chromatographic column packed with autoantibodies, through the affinity between autoantibody and autoantigen, and the extract of disease-related cell lines or pathological tissues, whereby an autoantigen screening platform is established.
Owner:IND TECH RES INST
Methods for screening antibodies
ActiveUS20120322686A1Library screeningPeptide preparation methodsAntibody conjugateAntibody screening
Owner:SEAGEN INC
Antibodies of coronavirus or antigen binding fragments of antibodies
ActiveCN112159469AImprove bindingStrong neutralization abilityImmunoglobulins against virusesAntiviralsAntigen Binding FragmentReceptor
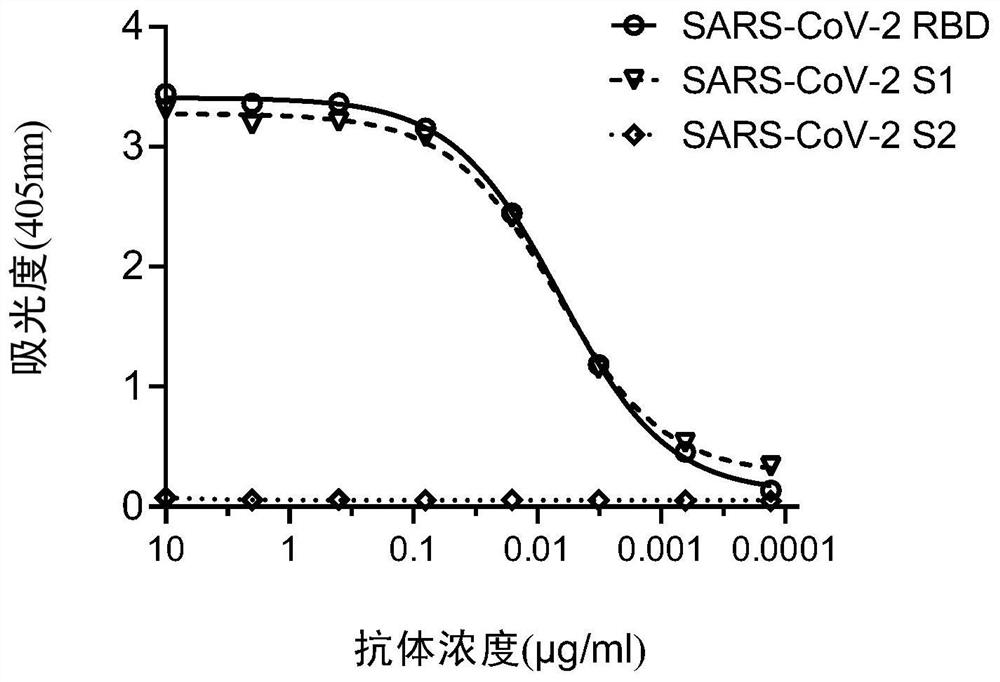
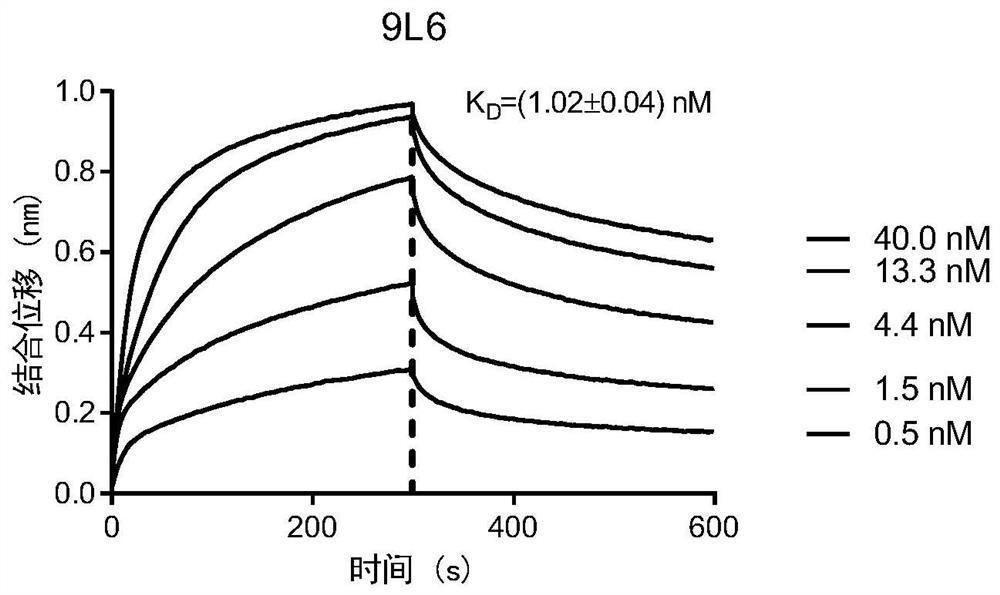
The invention relates to antibodies of a coronavirus or antigen binding fragments of the antibodies, a nucleic acid molecule for encoding the antibodies or the antigen binding fragments thereof, a vector including the nucleic acid molecule, a host cell including the vector, and application of the antibodies or the antigen binding fragments thereof to the aspect of preparation of drugs for treatingor preventing diseases caused by the coronavirus, and the aspect of detection products. The inventors utilize a B cell in-vitro monoclonal culture and high-throughput antibody screening technology toobtain a series of antibodies of the coronavirus and the antigen binding fragments of the antibodies, and the antibodies and the antigen binding fragments thereof have high binding capacity and neutralizing capacity for the SARS-CoV-2 virus, can recognize and bind S1 protein of the SARS-CoV-2 virus and a receptor binding domain (RBD) of the S1 protein, and have very strong affinity, and thus, itcan be speculated that the antibodies and the antigen binding fragments possibly have the binding capacity and neutralizing capacity for other coronaviruses, and coronaviruses possibly appearing in the future, and the antibodies and the antigen binding fragments have good clinical application prospects in the future.
Owner:超非凡(上海)医疗科技有限公司
Antibody screening methods
ActiveUS20140113831A1Improve propertiesQuick buildPeptide librariesSugar derivativesAntigen bindingAntibody screening
Provided are methods and compositions for the production of novel antibodies that bind specifically to a target anti gen. These methods and compositions are particularly useful for producing antibodies having the antigen binding specificity of a reference antibody but with improved properties (e.g., binding affinity, immunogenicity, and thermodynamic stability) relative to the reference antibody.
Owner:X BODY
Method of treating recurrent miscarriages
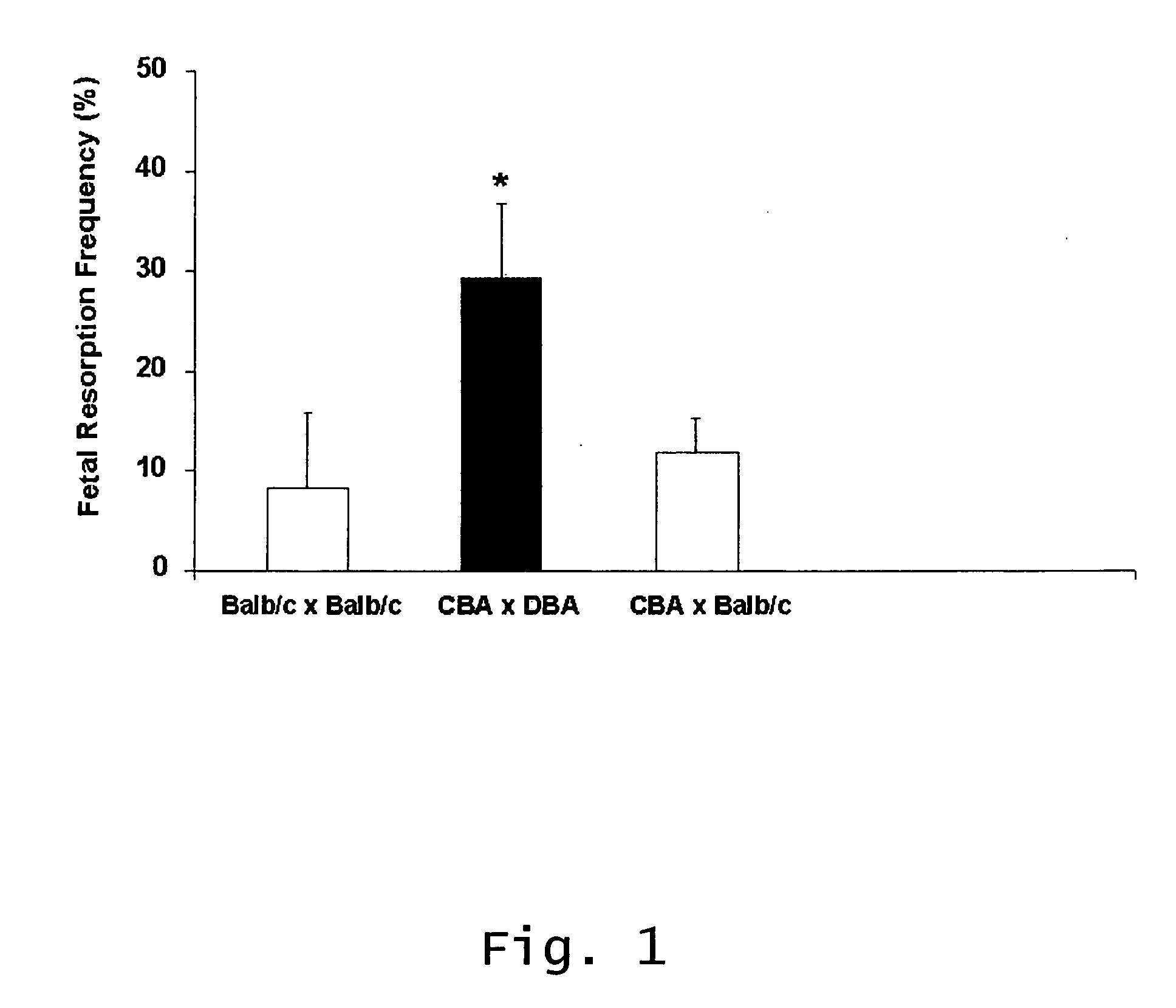
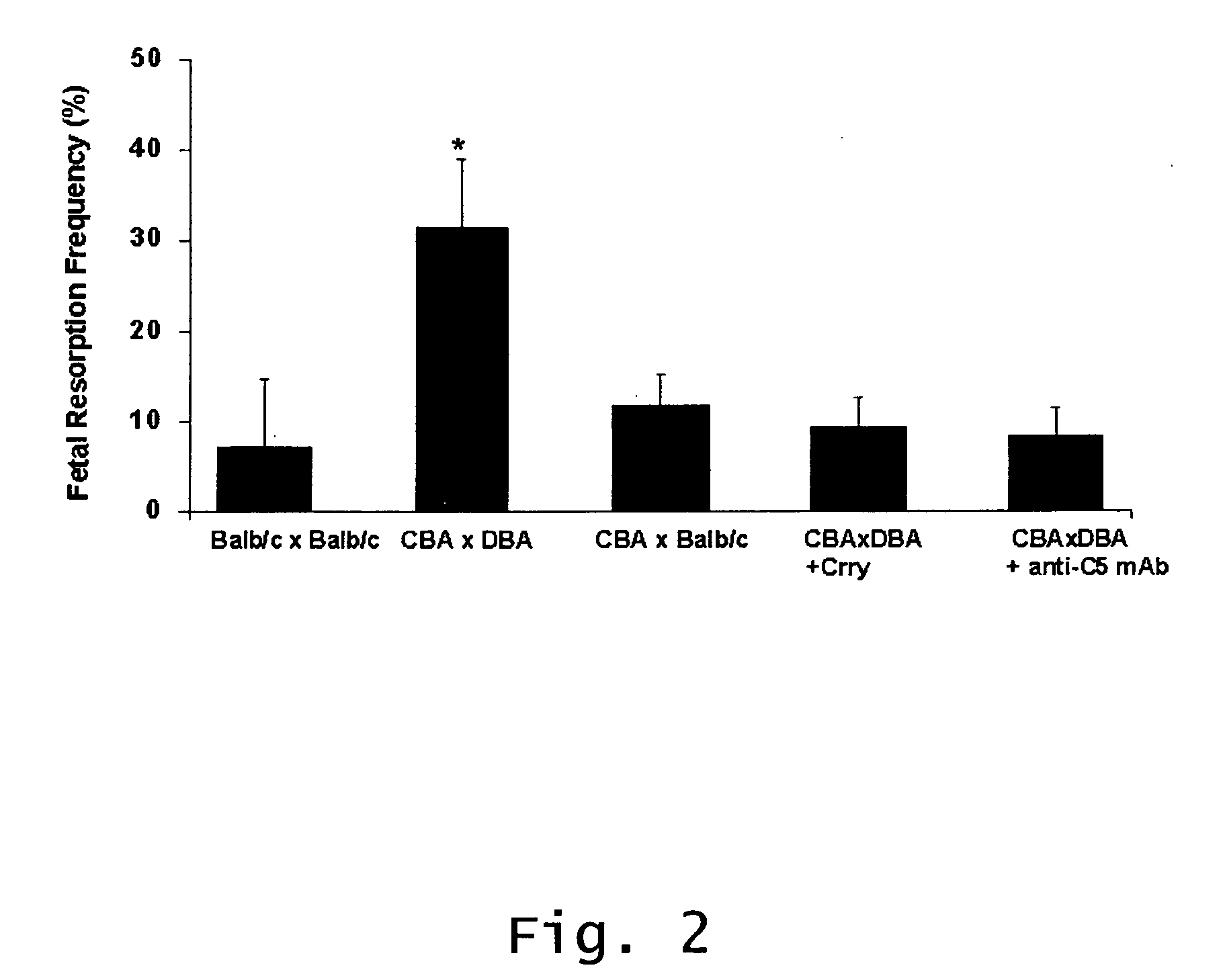
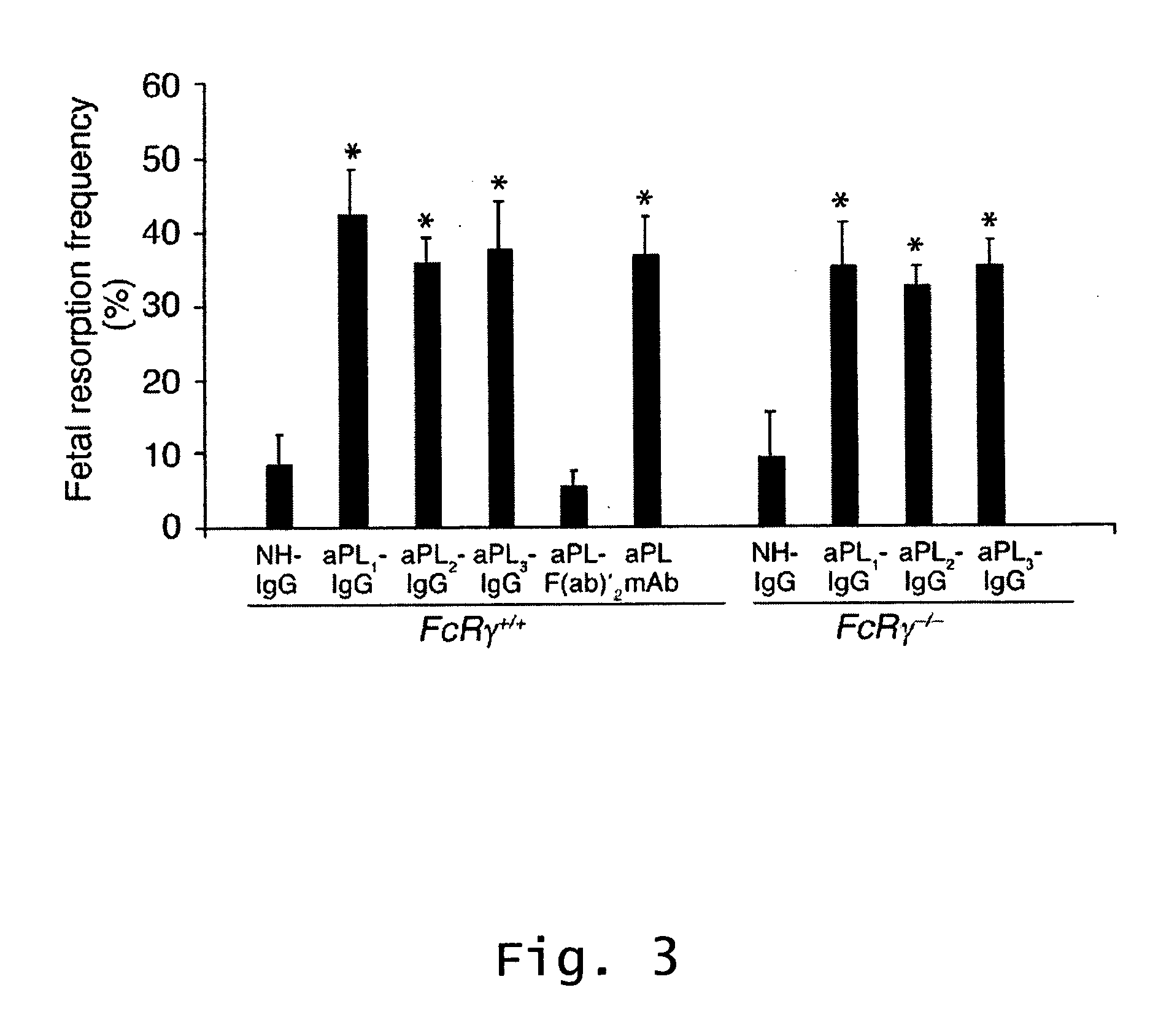
InactiveUS20070123466A1Prevent and reduce riskCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsRecurrent miscarriageScreening method
Described are methods for treating, preventing, or reducing the risk of, miscarriages, especially recurrent miscarriages. The methods comprise administering to a female subject a therapeutic agent that modulates the activity or binding of components of the complement system, together with a pharmaceutically acceptable carrier. For example, the therapeutic agent can be a C3-convertase inhibitor, an antibody against C5, an antagonist of the C5a receptor, or an antibody against factor B or factor D. Screening methods for agents that can prevent or reduce the risk of miscarriages, especially recurrent miscarriages, are also described.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY +1
Building method and application of antibody library
ActiveCN109576292AIncrease diversityImprove stabilityAntibody mimetics/scaffoldsMicrobiological testing/measurementPolyclonal antibodiesAntibody screening
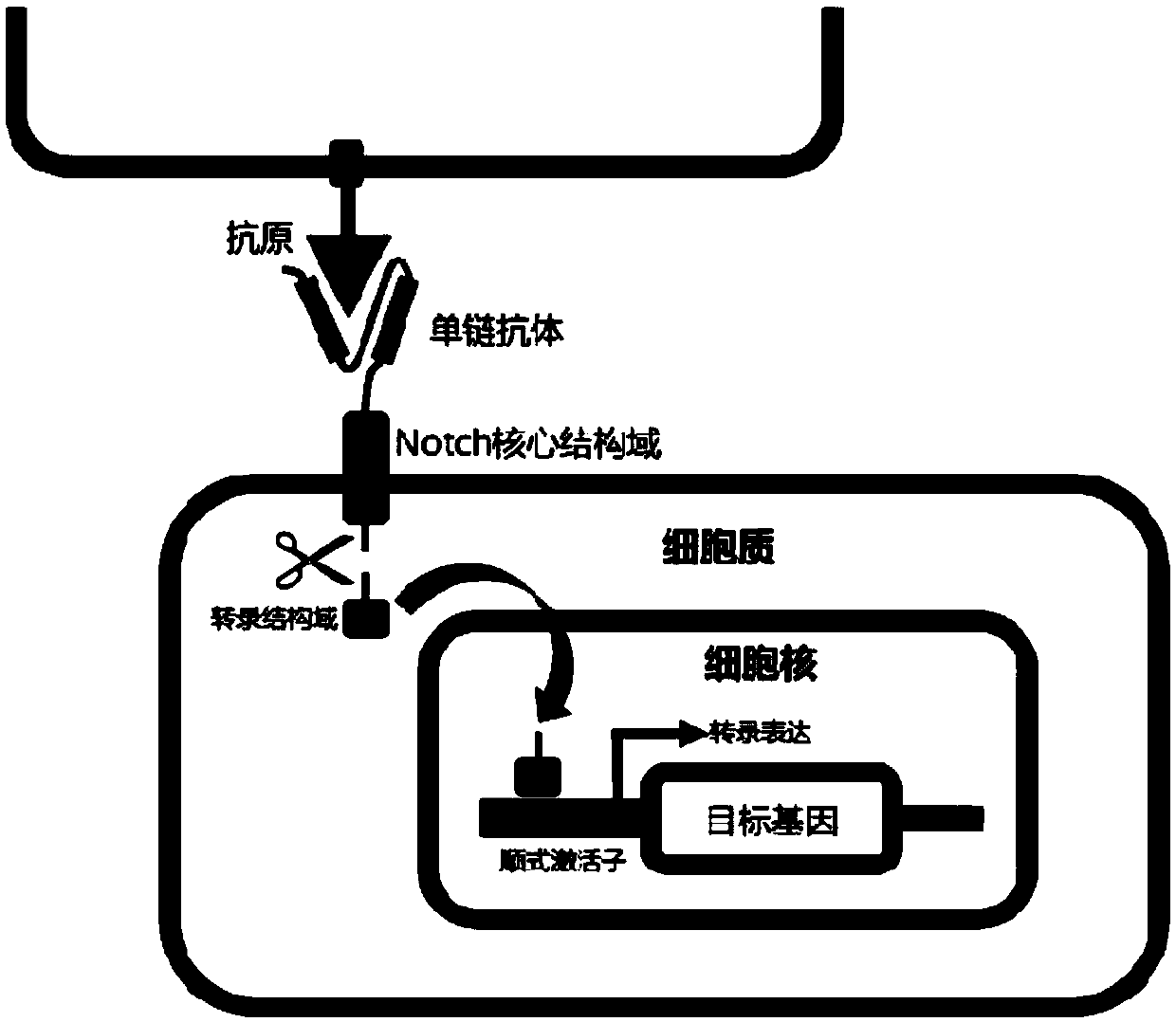
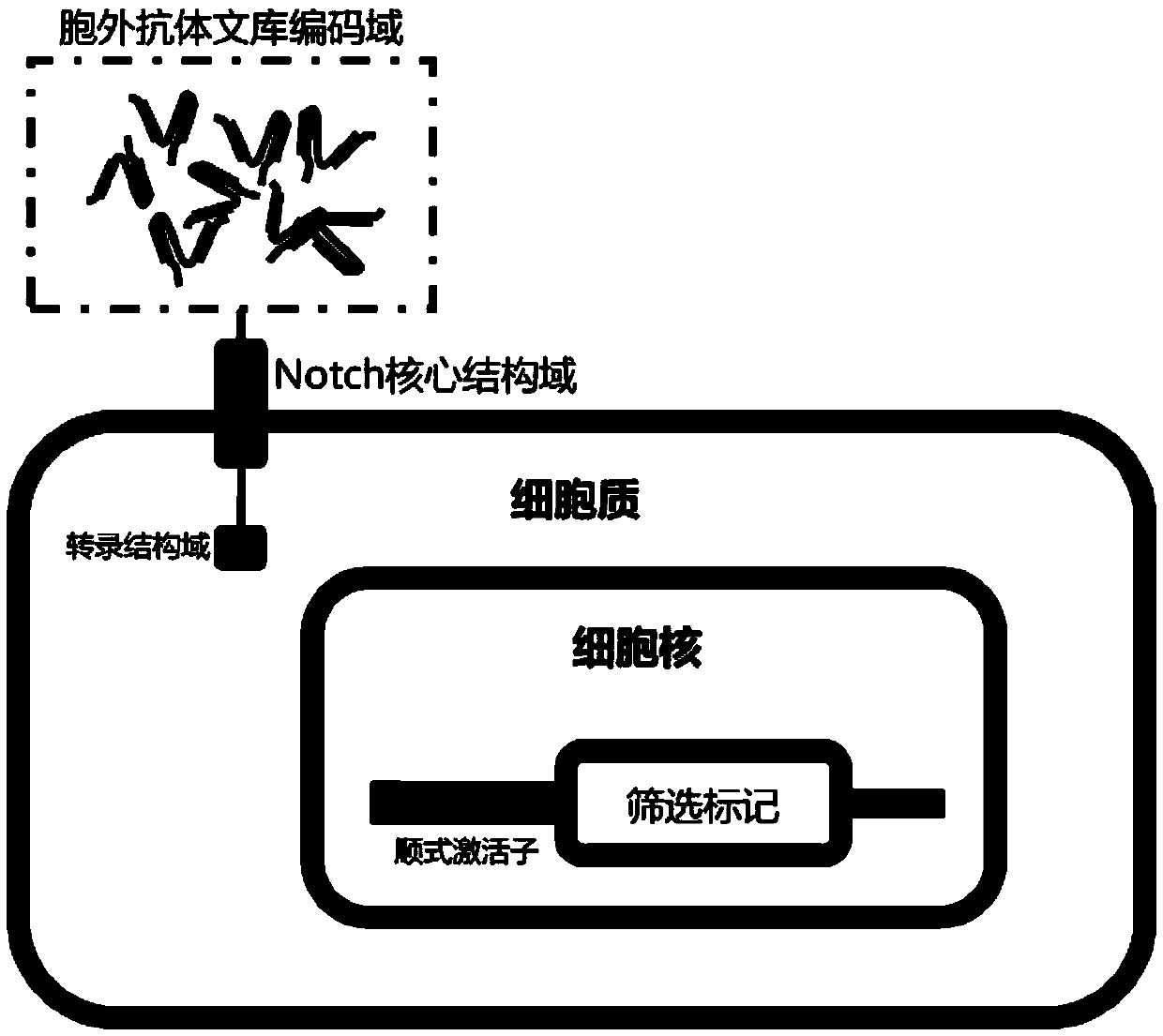
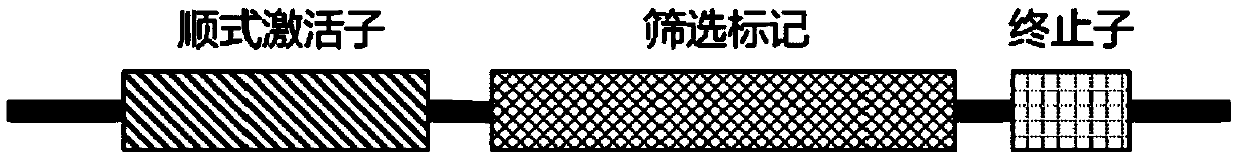
The invention provides a building method and application of an antibody library. The method is designed on the theory that a synNotch system controls the gene expression in cells; through groping by alarge number of experiments, the whole scheme flow process is optimized; through repeated design and verification, the extracellular recognition structural domain of the synNotch system is changed into the antibody library; a regulated and controlled target gene is changed into a screened marking gene, so that a simple, compact and efficient antibody screening method with various advantages is obtained; a polyclone antibody technology aiming at complex antigen can be screened; the problems of tumor antigen complexity, diversity, mutability and limited available targets are solved; wide application prospects and huge market values are realized.
Owner:SHENZHEN ISTIRBIO CO LTD
Aav vector and assay for Anti-aav (adeno-associated virus) neutralizing antibodies
ActiveUS20160123990A1Microbiological testing/measurementGenetic material ingredientsAnti virusViral antibody
Virus vectors, virus particles, and methods and uses of screening for, detecting, analyzing and determining amounts of virus antibody, or neutralizing antibody activity of samples are provided. Such virus vectors, virus particles, and methods and uses are applicable to a broad range of virus types, such as lentiviruses, adenovirus, and adeno-associated virus (AAV) serotypes. Methods and uses include virus antibody screening, such as anti-virus immunoglobulins screened for, detected, analyzed and amounts determined
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
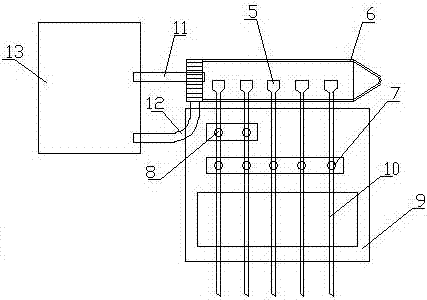
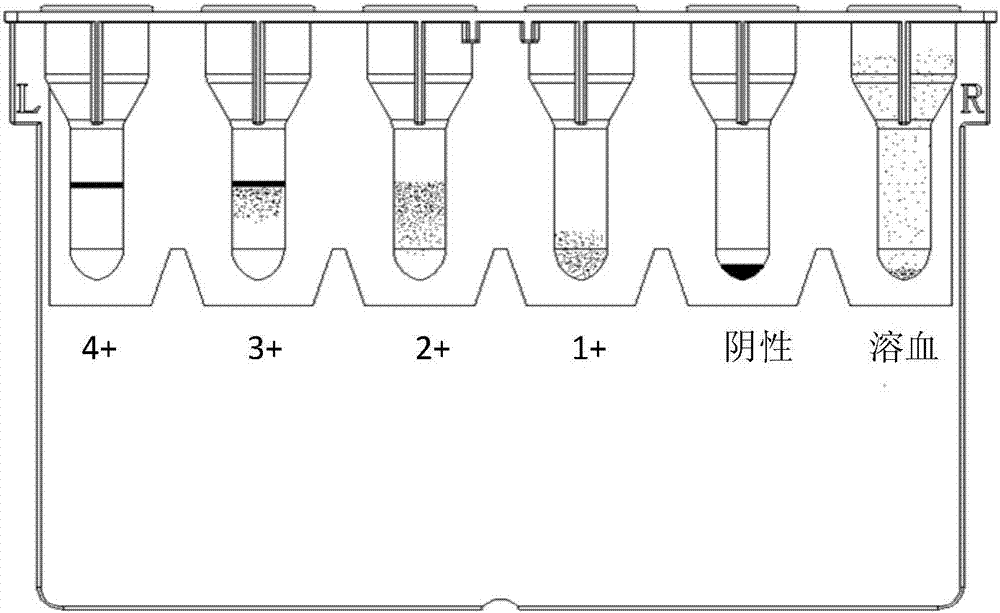
Cross-matching of blood, blood types A, B, O, and Rh D, and irregular antibody screening sample-adding machine
ActiveCN107247154AEasy to take pictures, scan filesSimple structureMaterial analysisGroup A - bloodBlood compatibility test
The invention relates to the technical field of medical experimental apparatus. Provided are cross-matching of blood, blood types A, B, O, and Rh D, and irregular antibody screening sample-adding machine. The screening sample-adding machine is provided with a distribution pipe with both ends sealed and characterized in that at least one injector is arranged side by side on the distribution pipe; needle parts of the injectors penetrating the distribution pipe are located at the lower end of the distribution pipe; radial through holes are arranged on the injectors inside the distribution pipe; a sample-adding pipe is arranged on the distribution pipe next to each injector; the sample-adding pipes are communicated with the injectors through the distribution pipe. The sample-adding machine solves the problems that sample-adding machines special for cross-matching of blood, blood types A, B, O, and Rh D, and irregular antibody screening experiments do not exist and it is complex, inefficient, and error-prone to operate with ordinary straw or dropper. cross-matching of blood, blood types A, B, O, and Rh D, and irregular antibody screening sample-adding machine are provided.
Owner:刘大基
Specific antibody of coronavirus, or antigen-binding fragment of specific antibody
ActiveCN112125973AHigh affinityImmunoglobulins against virusesAntiviralsAntigen Binding FragmentPharmaceutical drug
The invention relates to a specific antibody of coronavirus or an antigen-binding fragment of the specific antibody, a nucleic acid molecule encoding the antibody or the antigen-binding fragment thereof, a vector comprising the nucleic acid molecule, a host cell comprising the vector, an application of the antibody or the antigen-binding fragment thereof in the preparation of drugs for treating orpreventing diseases caused by coronavirus, and an application of the antibody or the antigen-binding fragment thereof in detection products. The antibody of coronavirus and the antigen-binding fragment of the antibody, which are obtained by inventors through the B cell in-vitro monoclonal culture and high-throughput antibody screening technology, have strong binding capacity and neutralizing capacity on the SARS-CoV-2 virus, can recognize and bind the S1 protein of the SARS-CoV-2 virus and the RBD of the S1 protein, and have very strong affinity. Therefore, it can be speculated that the antibody of coronavirus and the antigen binding fragment of the antibody possibly have binding capacity and neutralizing capacity on other coronaviruses and coronaviruses possibly occurring in the future,and thereby the antibody of coronavirus and the antigen binding fragment of the antibody have good clinical application prospects in the future.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Antibody screening method based on heavy chain library/light chain library of infectable virus particle type antibody and preparation method of heavy chain library/light chain library
ActiveCN103603057AHigh affinityStrong specificityLibrary screeningMicroorganism librariesSequence signalHeavy chain
The invention relates to a novel antibody screening method, the method comprises the following steps: (1) anti-body library construction: constructing a heavy chain library of infectable virus particle type antibody and a light chain library of infectable virus particle type antibody; (2) a first screening step: infecting animal cells with the heavy chain library of infectable virus particle type antibody and the light chain library of infectable virus particle type antibody, selecting the animal cells with a specific antibody expression ability by using a specific antigen so as to obtain positive cell strains; (3) a second screening step: obtaining the cDNA sequences of the heavy chain variable region and the light chain variable region of the specific antibody from the positive cell strains, inoculating light chain signal peptide sequence of the specific antibody, inserting an expression vector, and constructing an expression cell strain of the specific antibody so as to prepare the specific antibody through expression. The screening method has the advantages of simple and high efficient technology and practicality. The gene engineering antibody which is obtained through screening has a high specificity and high affinity.
Owner:SHENZHEN UNIV
Scaled procaryotic cell and eucaryotic cell internal antibody and antigen library construction and screening
InactiveCN1429916AHigh titerIncrease abundanceMicrobiological testing/measurementBiological testingEucaryotic cellDisease
A process for configuring multi-type, high-titer, high-abundance and high-diversity antibody libraries and antigen libraries in procaryotic cell and eukaryotic cell is disclosed, which features use of procaryotic and eukaryotic cell reproduction expression. Its screening features that according to the DNA sequence of antigen epitope gene, the all or part of the open reading frames for antigen gene and the special protein coding gene are combined to form fusion gene for screening the high-specificity and-affinity antibody.
Owner:韩泽广
Method of isolating a peptide which immunologically mimics microbial carbohydrates including group B streptococcal carbohydrates and the use thereof in a vaccine
This invention relates to new vaccines against microorganisms based on antigenically mimetic peptides. The invention also relates to methods of discovering such mimetic peptides by first screening peptide-display phage libraries with antibodies against the microbial carbohydrates(s) of interest to locate antigenically mimetic peptides. Vaccines against Group B Streptococcus, or Streptococcus Agalactiae, are preferably produced using this method.
Owner:MONTANA STATE UNIVERSITY
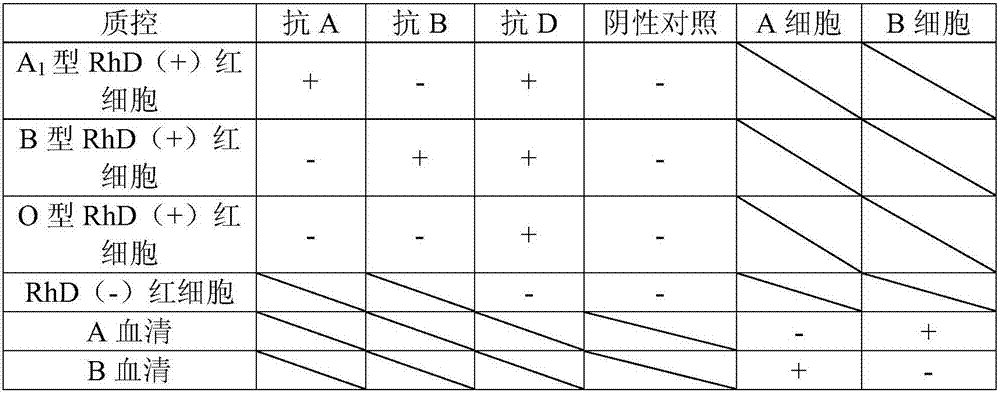
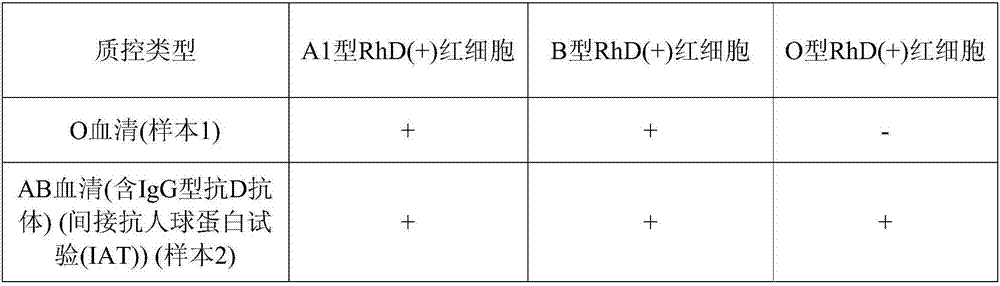
Blood type detection quality control product, preparation method thereof, and application thereof to blood type detection
The invention provides a blood type detection quality control product, a preparation method thereof, and application thereof to blood type detection. The blood type detection quality control product comprises 6 bottles of ABO, an RhD blood type detection quality control product, 5 bottles of cross-matching blood quality control products and 4 bottles of irregular antibody sieving test quality control products. The blood type detection quality control product can guarantee accuracy, specificity, affinity and stability of blood type detection, avoids influence on the detection result caused by unqualified detection in the detection process, and creates conditions for accuracy of ABO blood type determination, cross-matching blood and irregular antibody screening.
Owner:北京乐普诊断科技股份有限公司
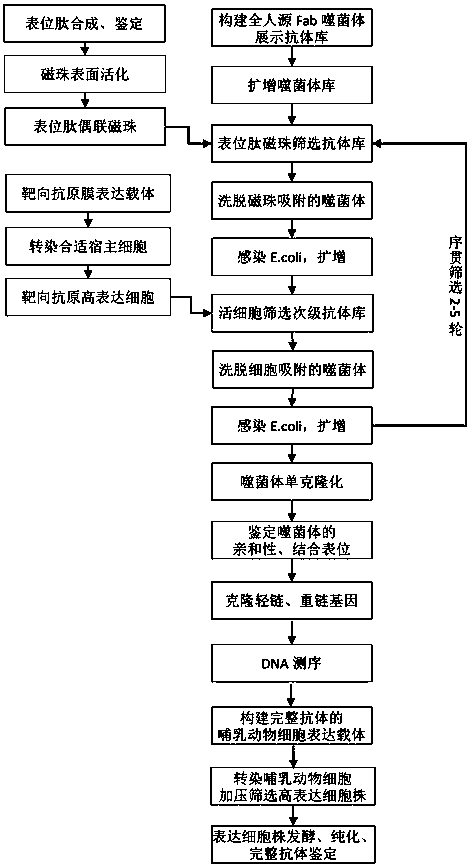
Epitope-specific antibody screening method and screened antibody
PendingCN109929036ASolve the problem of low hit rateSolve the problem of ineffective treatmentImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesDisease patient
The invention discloses an epitope-specific antibody screening method, and is used for solving the problem of low screening efficiency of functional antibodies having treatment function in an antibodylibrary screening technology; the method includes the steps of screening of functional epitope peptides and screening of living cells. The invention also provides a fully human anti-PD-1 monoclonal antibody obtained by screening a phage display antibody library by the method; for specific functional epitopes, the fully human anti-PD-1 monoclonal antibody can effectively block the binding of PD-1to a ligand of PD-1, has the characteristics of high affinity and low EC50, and can produce treatment effect on animal models at low dose. The screening efficiency of the functional antibody is greatly improved, and a potential, safer and more economical immunotherapy drug is provided for patients with malignant tumors and immune diseases.
Owner:TAIZHOU MABTECH PHARM CO LTD
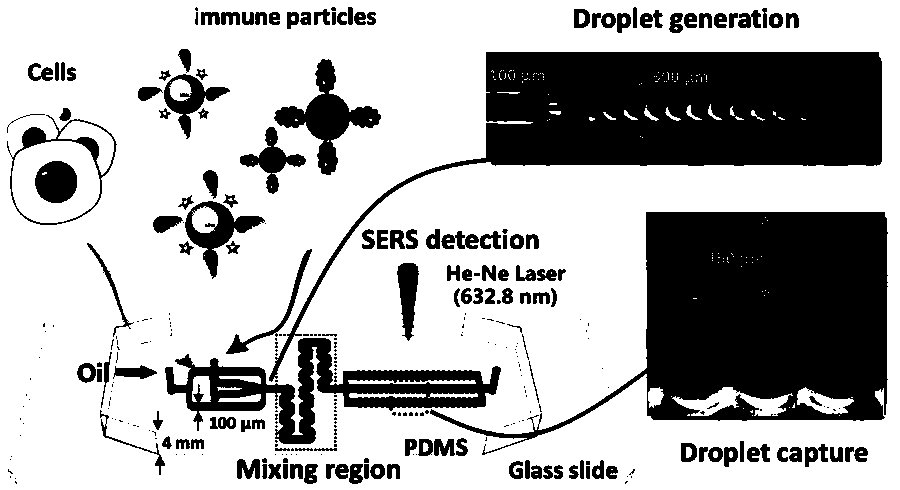
Preparation method of monoclonal antibody based on Raman spectroscopy and micro-droplet technology
The invention discloses a preparation method of a monoclonal antibody based on Raman spectroscopy and a microdroplet technology. The method comprises the steps of creating a micro-fluidic control platform and a corresponding detection probe and detecting the antibody content in microdroplets containing cells at high flux, wherein the microdroplets contain hybridoma cells formed by fusion of post-immune cells of an antibody immune animal or immune cells and myelomas. The ultra-efficient new technical method is provided for the preparation of the monoclonal antibody, the monoclonal screening cycle is shortened greatly, and the antibody screening capability is improved by geometric times.
Owner:梁重阳 +1
Method of isolating a peptide which immunologically mimics microbial carbohydrates including group B streptococcal carbohydrates and the use thereof in a vaccine
InactiveUS20030082524A1Bacterial antigen ingredientsMicrobiological testing/measurementMicroorganismStreptococcus agalactiae
This invention relates to new vaccines against microorganisms based on antigenically mimetic peptides. The invention also relates to methods of discovering such mimetic peptides by first screening peptide-display phage libraries with antibodies against the microbial carbohydrates(s) of interest to locate antigenically mimetic peptides. Vaccines against Group B Streptococcus, or Streptococcus Agalactiae, are preferably produced using this method.
Owner:MONTANA STATE UNIVERSITY
Antibody discovery method based on hybridoma technique and high-throughput sequencing
InactiveCN106065031AImprove the efficiency of structure optimizationExpand the scope of the filterImmunoglobulins against virusesImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody screeningHybridoma technology
The invention relates to a method for generating a recombinant antibody combining at least one target antigen and the recombinant antibody generated thereby. The antibody generation method provided by the invention combines an optimized high-throughput antibody sequencing method with a hybridoma antibody screening technology, and compared with the traditional hybridoma monoclonal antibody technology, enlarges the selection range of candidate antibody genes, improves the antibody structure optimization efficiency, and increases the possibility of acquiring functional antibodies.
Owner:GENEWIZ INC SZ
Antibody screening methods
ActiveUS9880160B2Improve propertiesQuick buildLibrary screeningImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody screeningAntigen binding
Provided are methods and compositions for the production of novel antibodies that bind specifically to a target antigen. These methods and compositions are particularly useful for producing antibodies having the antigen binding specificity of a reference antibody but with improved properties (e.g., binding affinity, immunogenicity, and thermodynamic stability) relative to the reference antibody.
Owner:X BODY
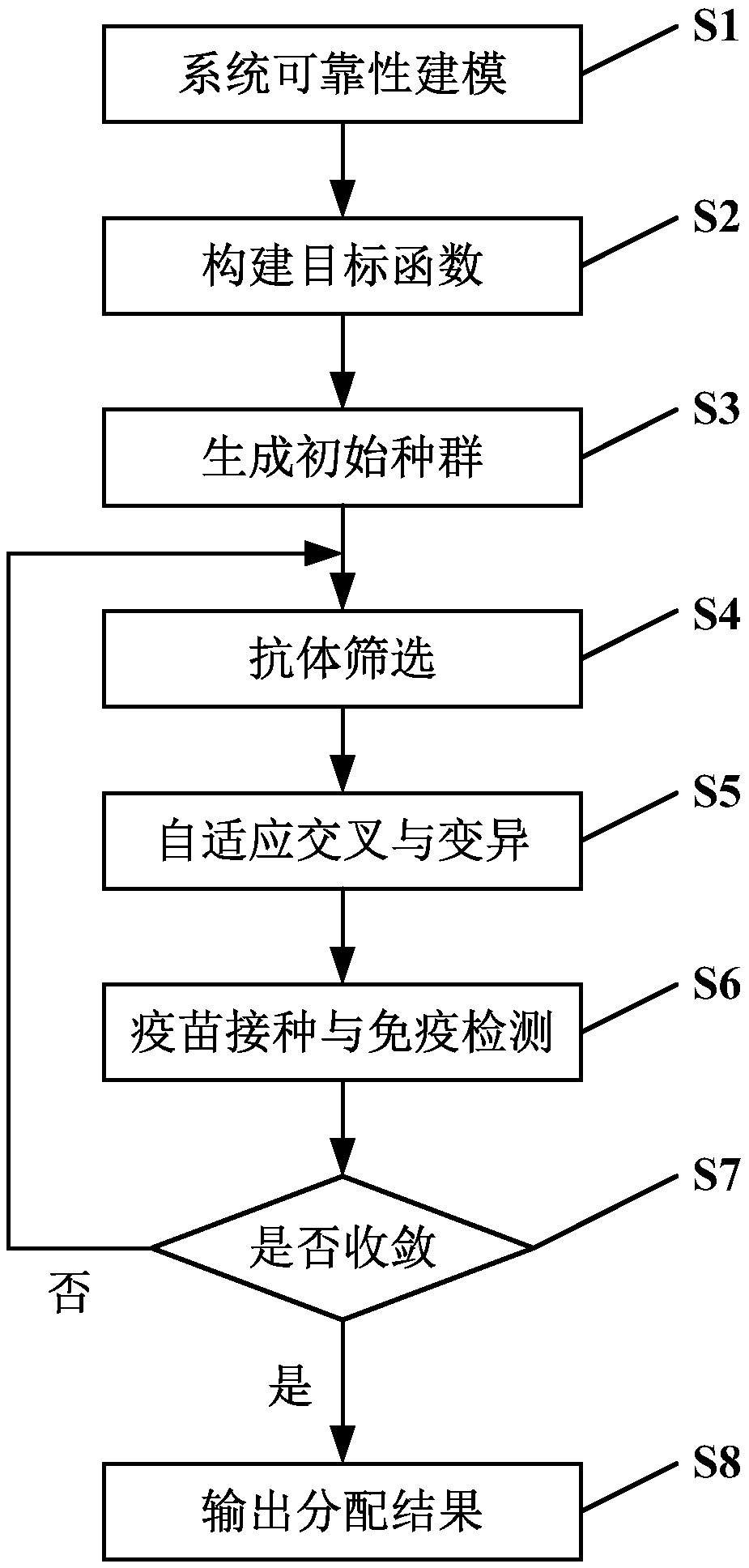
Reliability distribution method and apparatus based on immune genetic optimization
InactiveCN105512726AGuaranteed continuationLow lifetime costGenetic algorithmsDistribution methodGenetic algorithm
The invention discloses a reliability distribution method and apparatus based on immune genetic optimization, and relates to the field of reliability distribution. The method comprises the following steps: eliminating devices with selected models and devices with determined reliability indexes, and then according to operation working conditions of residual devices, carrying out system reliability modeling; constructing full-life cost function and obtaining a function taking the system full-life cost as an object; and randomly generating initial population, screening antibodies in the population, operating adaptive intersection and variation operation of a genetic algorithm, next, operating an immunization algorithm, extracting a vaccine, vaccinating the antibodies, then classifying the antibodies with higher fitness into new-generation population, determining whether the new-generation population is convergent, if so, outputting a distribution result, and otherwise, returning the antibody screening step. According to the invention, minimization of full-life cost is taken as a distribution object, the immunization algorithm and the genetic algorithm are combined together in a distribution process, and the method and apparatus provided by the invention are applied to task reliability distribution of a complex system.
Owner:NO 719 RES INST CHINA SHIPBUILDING IND
Antibody screening method based on single cell chip technology
InactiveCN107219351AQuick access to sequenceQuick access to affinity dataImmunoglobulins against animals/humansBiological testingSpleen cellFluorescence
The invention discloses an antibody screening method based on a single cell chip technology. The method comprises the following steps: 1) expressing a cellular immunized mouse with a special surface antigen and extracting a spleen cell of the mouse for ELISA analysis; 2) attaching the single cell to a cell chip according to the result, and combining the cell chip with fluorescently-labeled antigen to obtain positive clone information and affinity information of antibody; and 3) performing on-site gene amplification on the antibody meeting the standard to obtain an antibody variable region sequence. The method disclosed by the invention can quickly obtain a lot of sequence and affinity data of the antibody. The screening method disclosed by the invention lays a good foundation for pharmaceutical research field.
Owner:GUANGDONG INST OF APPLIED BIOLOGICAL RESOURCES
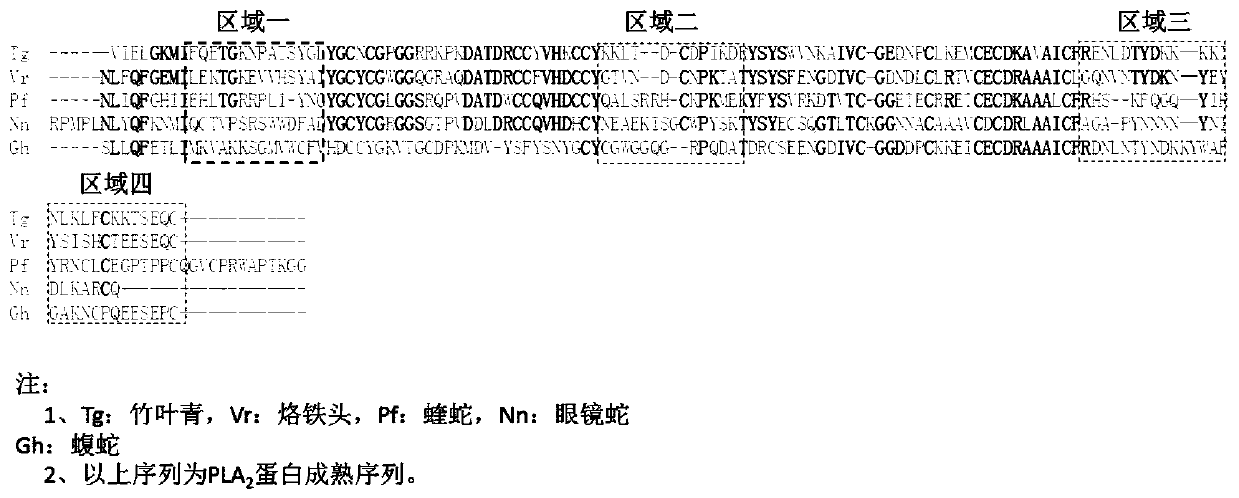
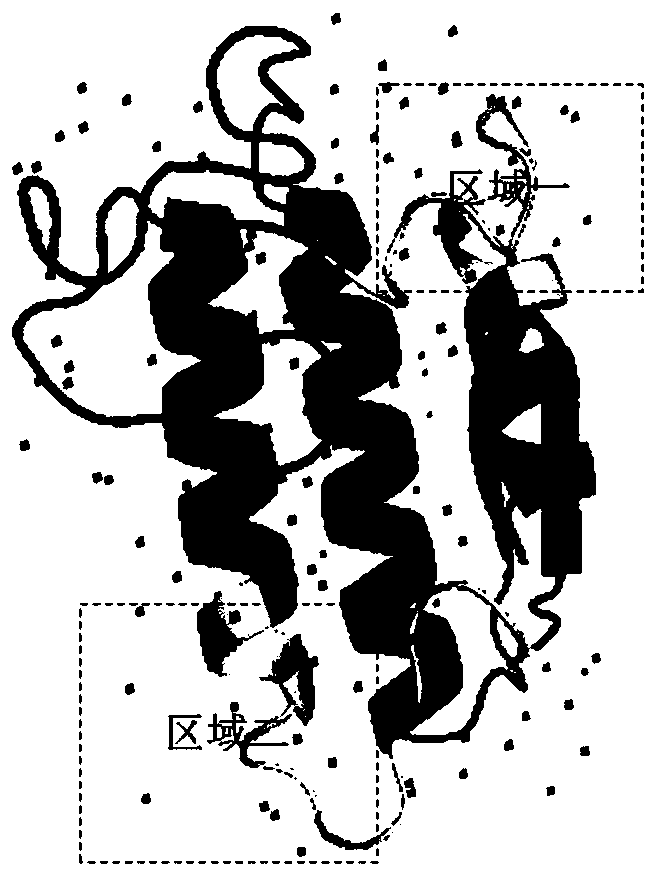
Anti-venomous-snake PLA2 protein antibodies and application thereof
InactiveCN110317270AImprove diagnostic efficiencyHigh affinityImmunoglobulins against animals/humansDisease diagnosisMonoclonal antibodyAntibody screening
The invention relates to anti-venomous-snake PLA2 protein antibodies and application thereof. The anti-venomous-snake PLA2 protein antibodies comprise monoclonal antibodies of tritoxomab-PLA2, protoxomab-PLA2, viptoxomab-PLA2, najatoxomab-PLA2 or glotoxomab-PLA2, and have high affinity and specificity. The monoclonal antibodies are prepared by adopting PLA2 proteins of the five kinds of venomous snakes as antigens for immunizing mice through a hybridoma fusion technology, antibody screening is conducted by taking the amino acid sequences of of two hypervariable regions in the PLA2 proteins asantigens, and finally, ten monoclonal antibodies of tritoxomab-PLA2, protoxomab-PLA2, viptoxomab-PLA2, najatoxomab-PLA2 and glotoxomab-PLA2 are obtained in total. One or a combination of more of the ten monoclonal antibodies can be used as a diagnostic test reagent for snake wounds. The anti-venomous-snake PLA2 protein antibodies can be prepared into the diagnostic reagent or kit for the snake wounds, and the diagnostic efficiency of the snake wounds is improved.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
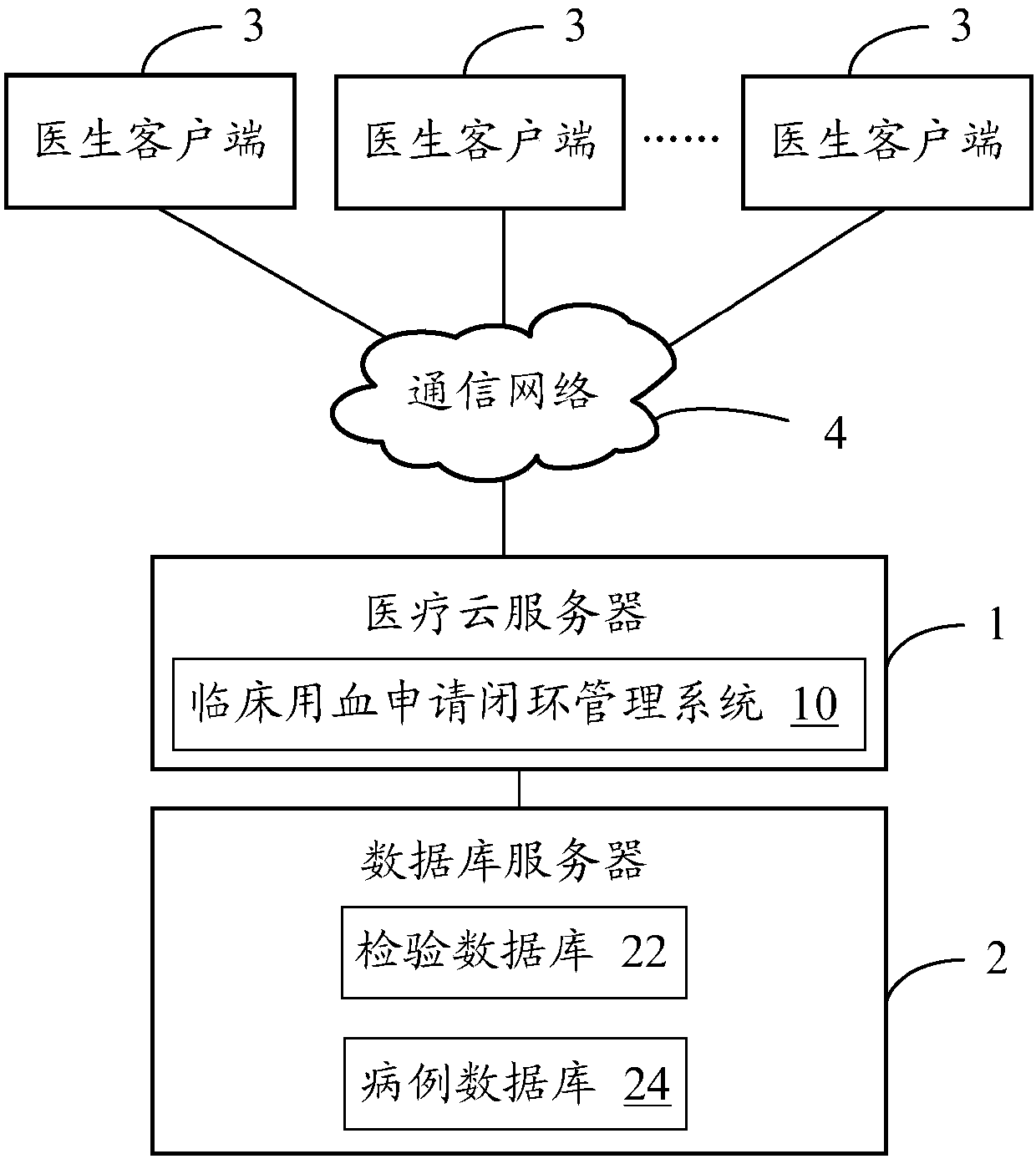
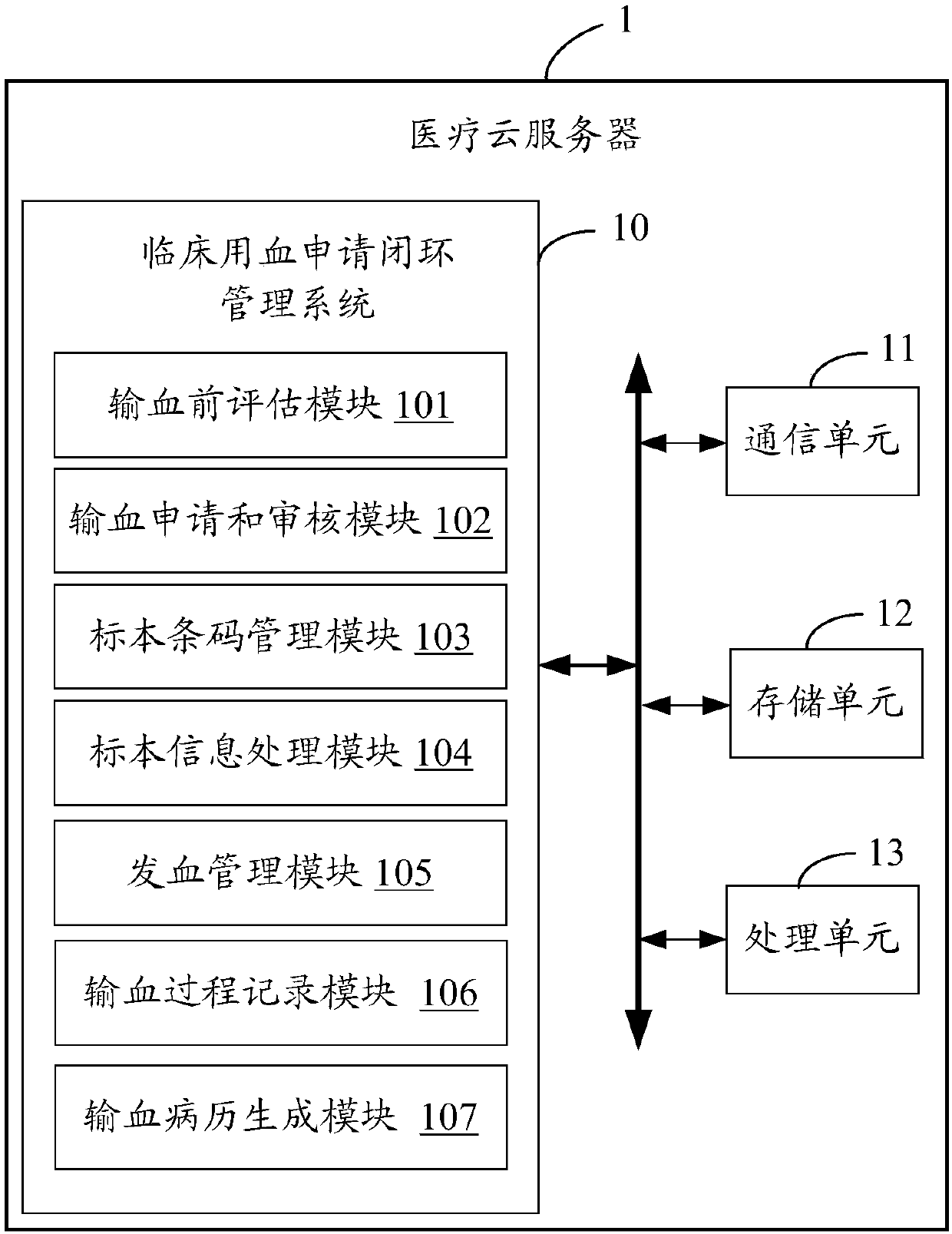
Clinical blood use application closed-loop management system and method
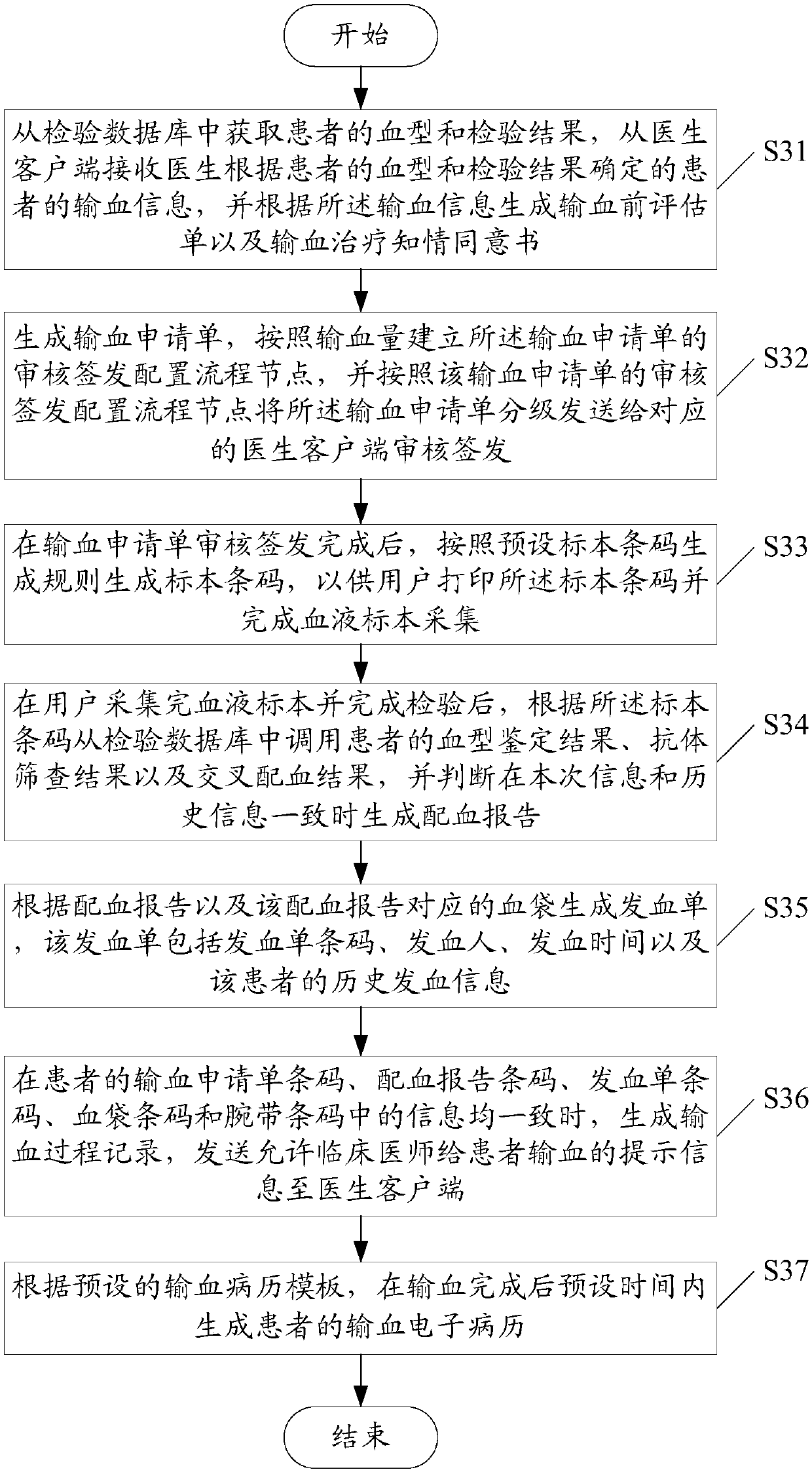
InactiveCN107563113AGuaranteed reasonablenessEnsure consistencySpecial data processing applicationsInformaticsMedical recordClosed loop
The invention provides a clinical blood use application closed-loop management system and method. The method comprises the steps of obtaining a blood type and a test result of a patient and receivingblood transfusion information of the patient from a doctor client; according to a blood transfusion volume, establishing an auditing, signing and issuing configuration process node of a blood transfusion application form and performing graded auditing, signing and issuing; according to a preset specimen barcode generation rule, generating a specimen barcode; according to the specimen barcode, calling a blood type identification result, an antibody screening result and a cross blood matching result of the patient, and when the current information is judged to be consistent with historical information, generating a blood transfusion report; according to the blood transfusion report and a blood bag corresponding to the blood transfusion report, generating a blood distribution form; generatinga blood transfusion process record; and according to a preset blood transfusion medical record template, generating a blood transfusion electronic medical record of the patient in a preset time afterblood transfusion completion. By implementing the method and the system, clinical blood use process information traceability can be ensured, so that clinical blood use reasonability is improved.
Owner:ANYCHECK INFORMATION TECH
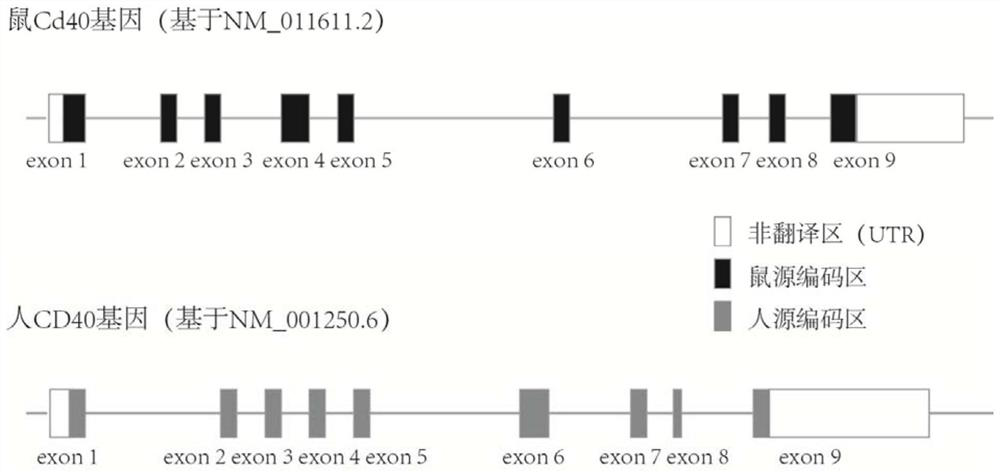
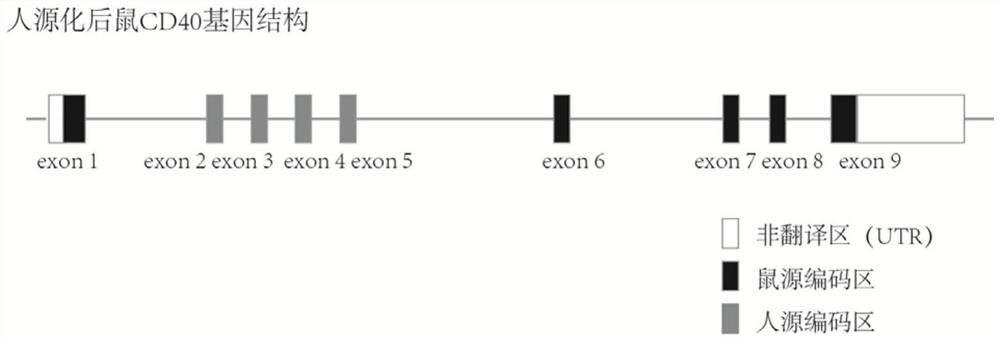
Construction method of humanized CD40 gene modified animal model and application thereof
PendingCN112725379AReduce development riskSpeed up the R&D processNGF-receptor/TNF-receptor superfamilyFermentationDiseaseTumor therapy
The invention provides a construction method of a humanized CD40 gene modified animal model and an application thereof, and relates to the field of gene engineering. The CD40 gene humanized model animal is successfully prepared, the humanized CD40 protein can be normally expressed in the model body, and the product can be used for CD40 gene function research and humanized CD40 antibody screening and evaluation. The animal model prepared by the method can be applied to drug screening, drug effect research, immune related diseases, tumor treatment and the like for human CD40 target sites, the research and development process of new drugs is accelerated, the time and cost are saved, and the drug development risk is reduced. A powerful tool is provided for researching the function of the CD40 protein and screening tumor drugs.
Owner:SHANGHAI BIOMODEL ORGANISM SCI & TECH DEV +2
Electronic blood matching method
InactiveCN103745099ASimplify the blood matching processSpecial data processing applicationsAntibody screeningBiomedical engineering
The invention discloses an electronic blood matching method and relates to a technology of medical treatment information diagnosis. Confirmation and information recording are conducted twice on blood types of a blood donator and a patient to sufficiently confirm the matching condition of the blood types of the blood donator and the patient, antibody screening experiments are conducted on the blood donator and the patient to further ensure blood type matching safety and accuracy, and the method greatly improves blood matching efficiency.
Owner:CHONGQING TOP INFORMATION TECH +1
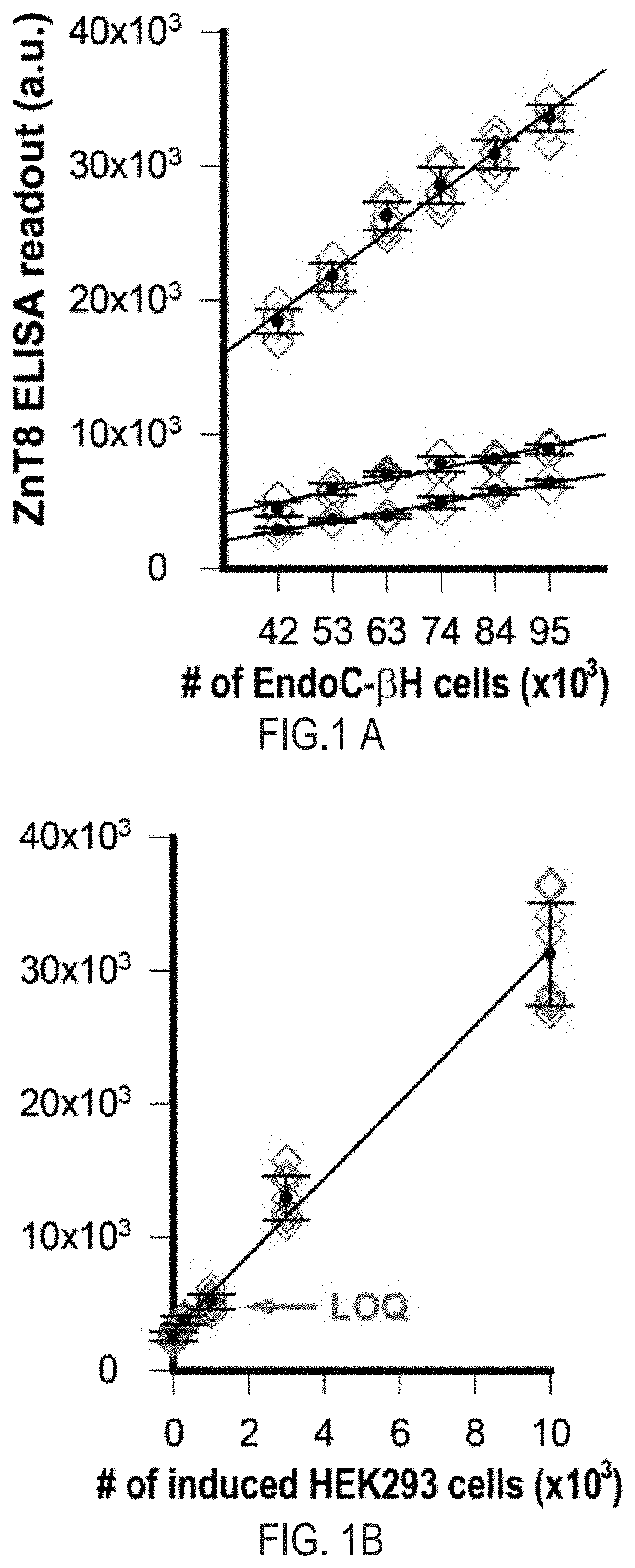
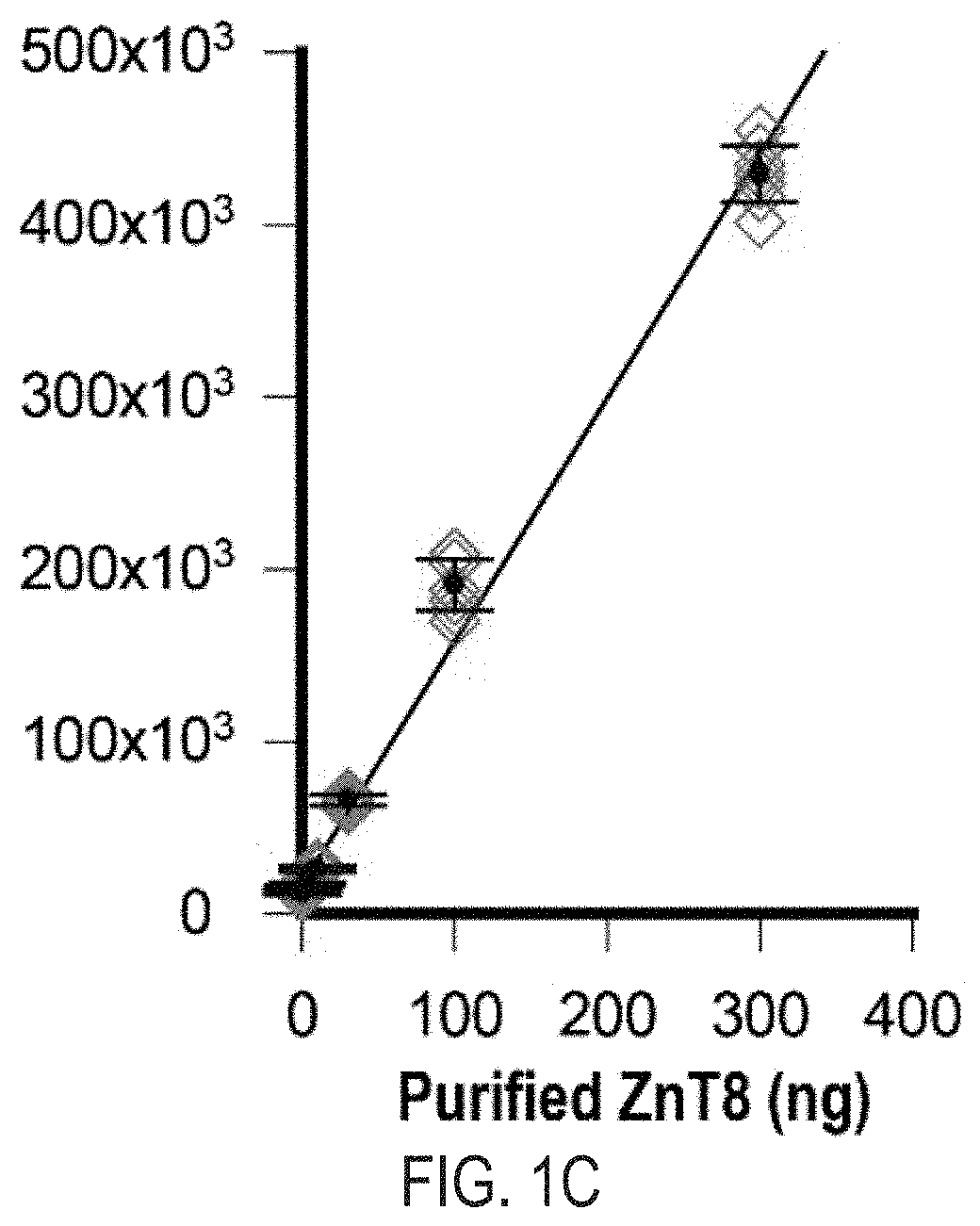
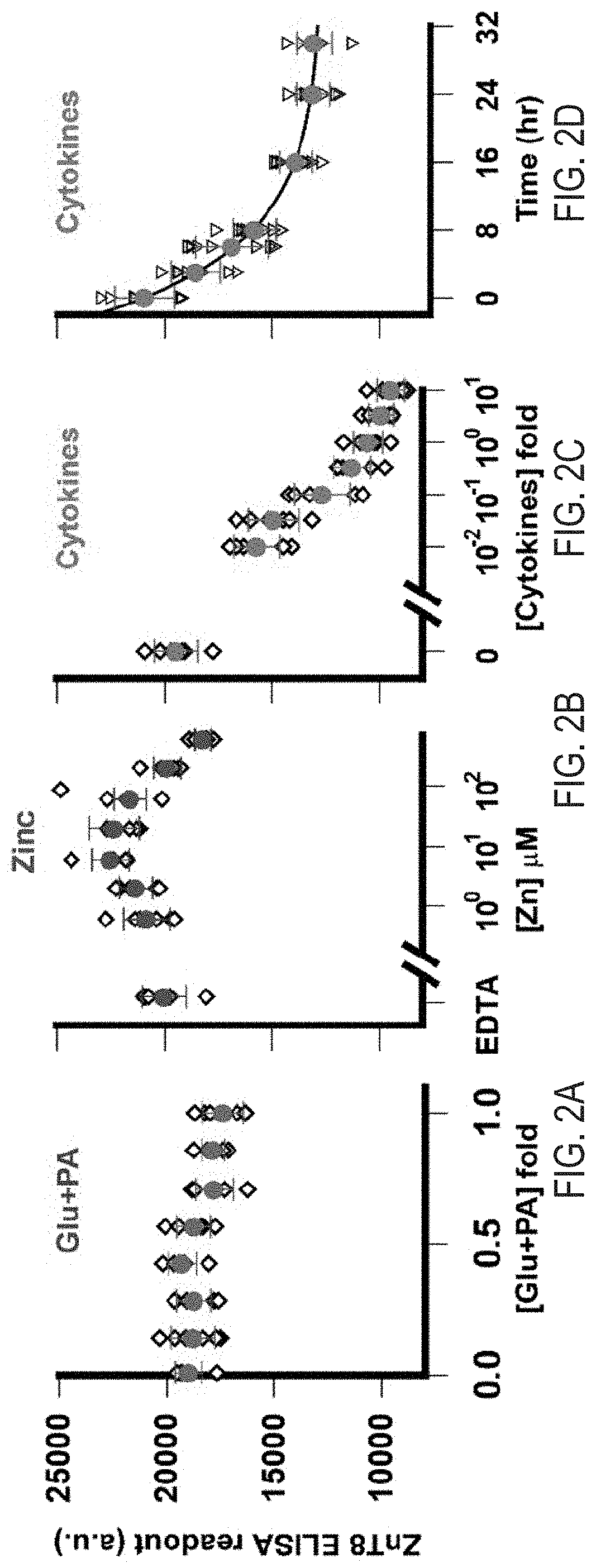
Cell-based znt8 assay
PendingUS20210302413A1Immunoglobulins against cell receptors/antigens/surface-determinantsBiological testingDiabetes mellitusAssay
The present invention relates to the fields of immunology and diabetes. More specifically, the present invention provides methods and compositions directed to the use of antibodies to quantify cellular pancreatic zinc transporter 8. In certain embodiment, the present invention provides methods and compositions directed to the use of antibodies to screen for modulators of the pancreatic zinc transporter, ZnT8. In one embodiment, a method comprises the steps of (a) permeabilizing human beta cells present in a substrate; (b) contacting the cells with a test agent; and (c) measuring the amount of zinc transporter 8 (ZnT8) using at least one anti-ZnT8 antibody or antigen-binding fragment thereof.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
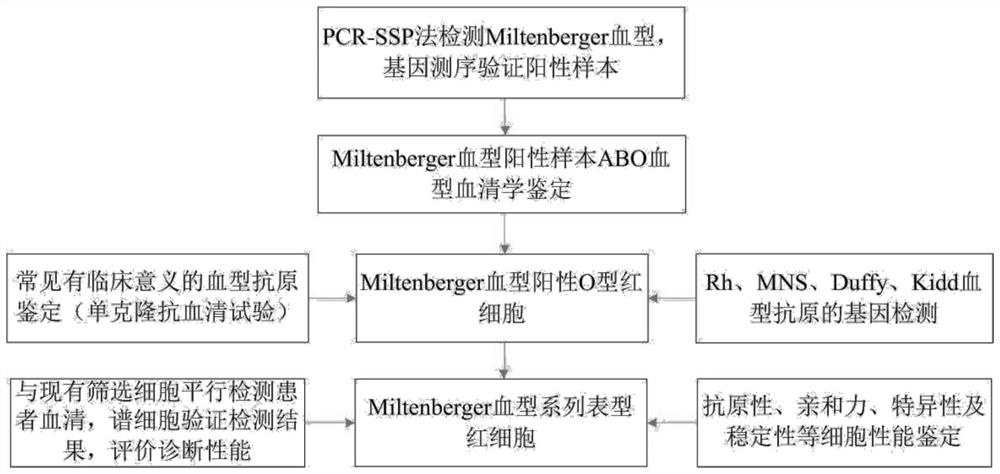
Preparation method of Miltenberger blood group series phenotype red blood cells
PendingCN112126623AAvoidance of hemolytic transfusion reactionsAvoid ineffective blood transfusionMicrobiological testing/measurementBlood/immune system cellsRed blood cellAntibody screening
The invention discloses a preparation method of Miltenberger blood group series phenotype red blood cells. The preparation method includes the following steps: step one, adopting monoclonal antibodiesto identify common clinically significant Rh, Kidd, MNS, Duffy, Lewis and P system antigens on Miltenberger blood group positive type O red blood cells; step two, respectively performing PCR-SSP andgene sequencing validation on dose effect antigen C, c, E, e, M, N, S, s, Fya, Fyb, Jka and Jkb to obtain a red blood cell surface antigen pattern; step three, using a red blood cell reagent preservation solution to prepare into a Miltenberger blood group series phenotype red blood cell reagent; and step four, using the prepared Miltenberger blood group series phenotype red blood cell reagent andan existing irregular antibody screening cell reagent to parallelly detect the serum of a hospitalized blood transfusion patient, vertifying the diagnostic performance of the cell reagent, and obtaining a final product. Through the cooperation of the prepared Miltenberger blood group series phenotype red blood cells and the existing screening cells, detection can be performed on Miltenberger bloodgroup related antibodies, and therefore, safe blood transfusion can be guaranteed.
Owner:南昌大学第一附属医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com