Patents
Literature
112 results about "Small cell lung carcinoma cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
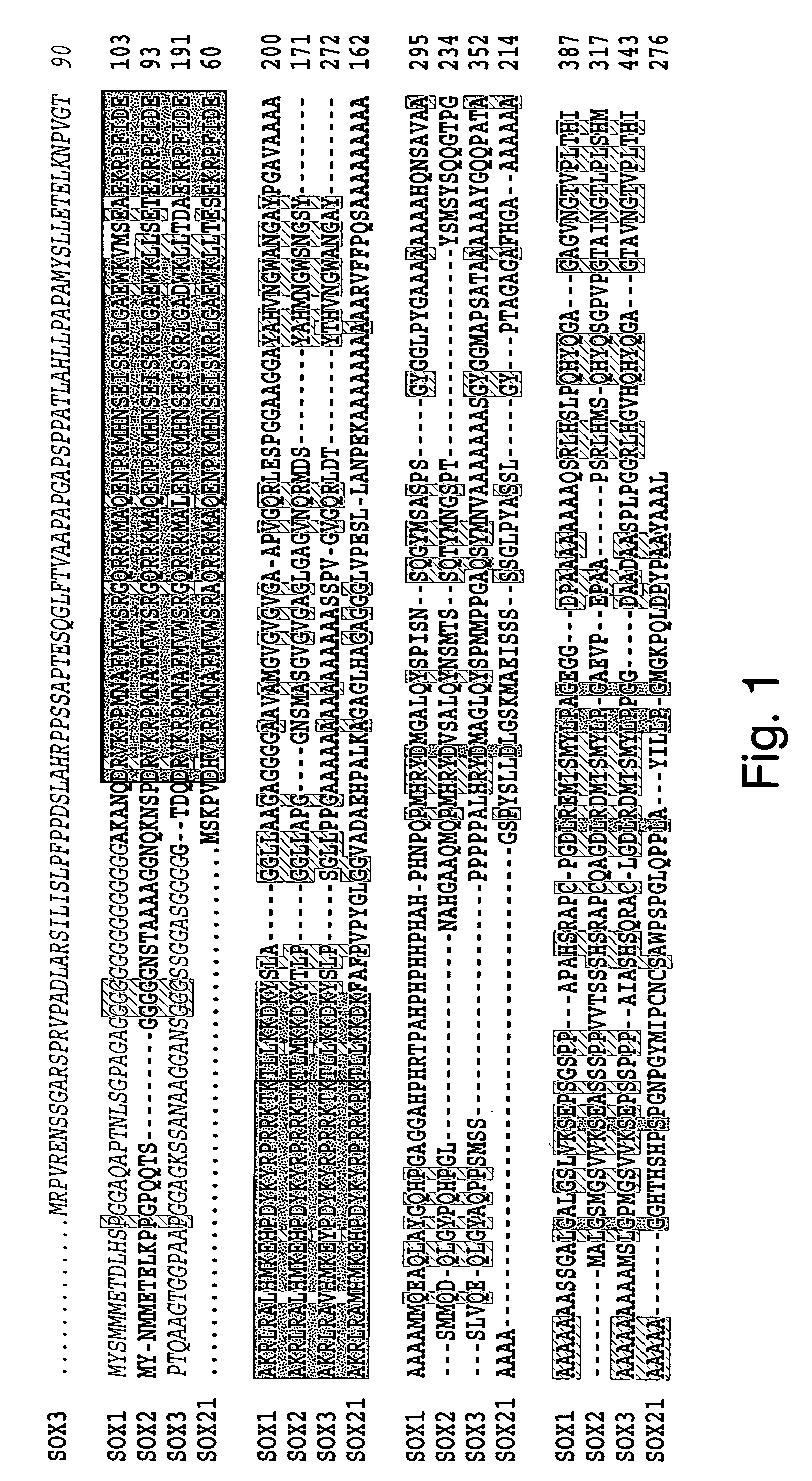
Small cell lung cancer associated antigens and uses therefor
InactiveUS7314721B2Tumor rejection antigen precursorsPeptide/protein ingredientsSerum igeADAMTS Proteins
Cancer associated antigens have been identified by autologous antibody screening of libraries of nucleic acids expressed in small cell lung cancer cells using antisera from cancer patients. The invention relates to nucleic acids and encoded polypeptides which are cancer associated antigens expressed in patients afflicted with small cell lung cancer. The invention provides, among other things, isolated nucleic acid molecules, expression vectors containing those molecules and host cells transfected with those molecules. The invention also provides isolated proteins and peptides, antibodies to those proteins and peptides and cytotoxic T lymphocytes which recognize the proteins and peptides. Fragments of the foregoing including functional fragments and variants also are provided. Kits containing the foregoing molecules additionally are provided. The molecules provided by the invention can be used in the diagnosis, monitoring, research, or treatment of conditions characterized by the expression of one or more cancer associated antigens.
Owner:NEW YORK HOSPITAL CORNELL MEDICAL CENT +2
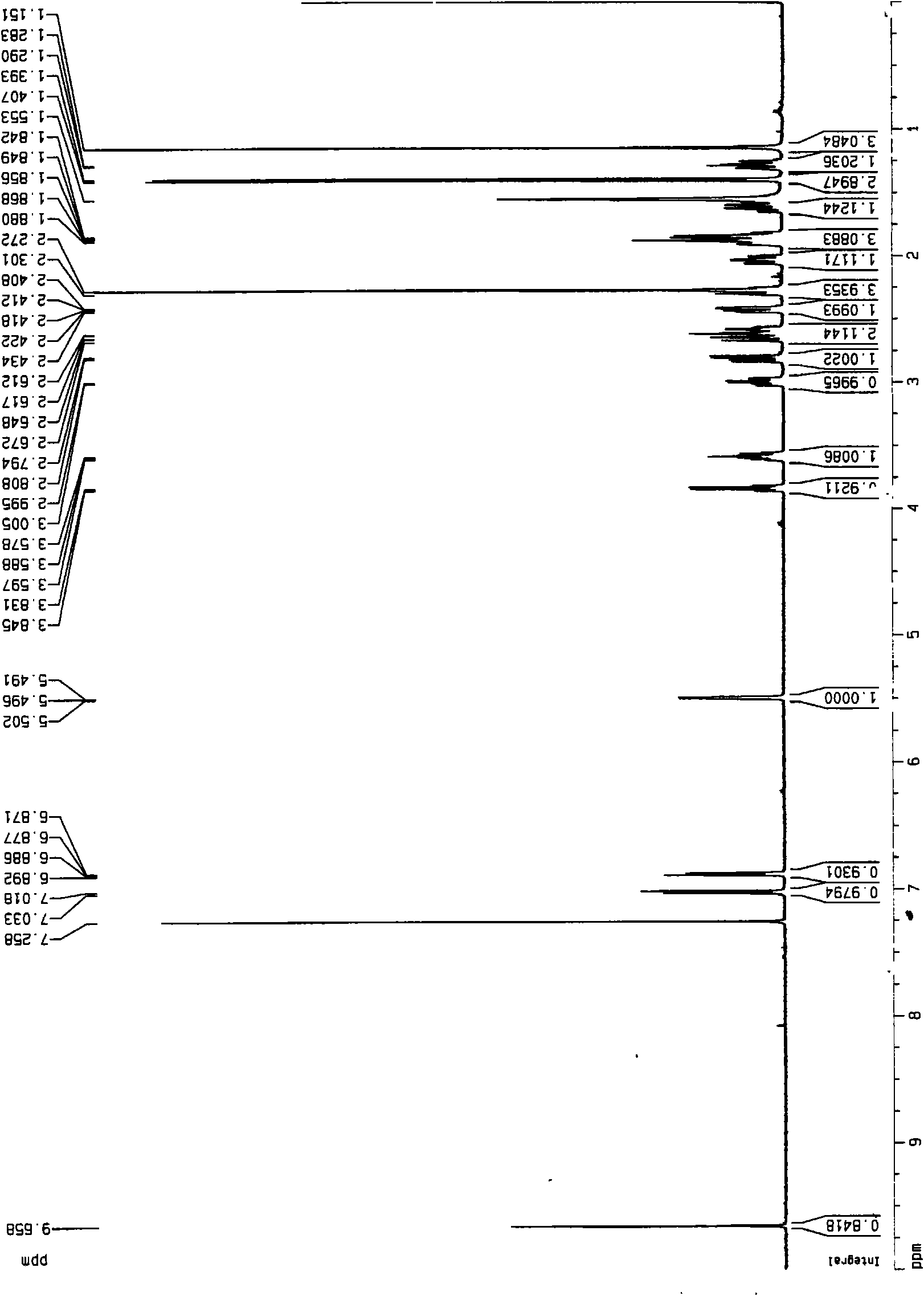
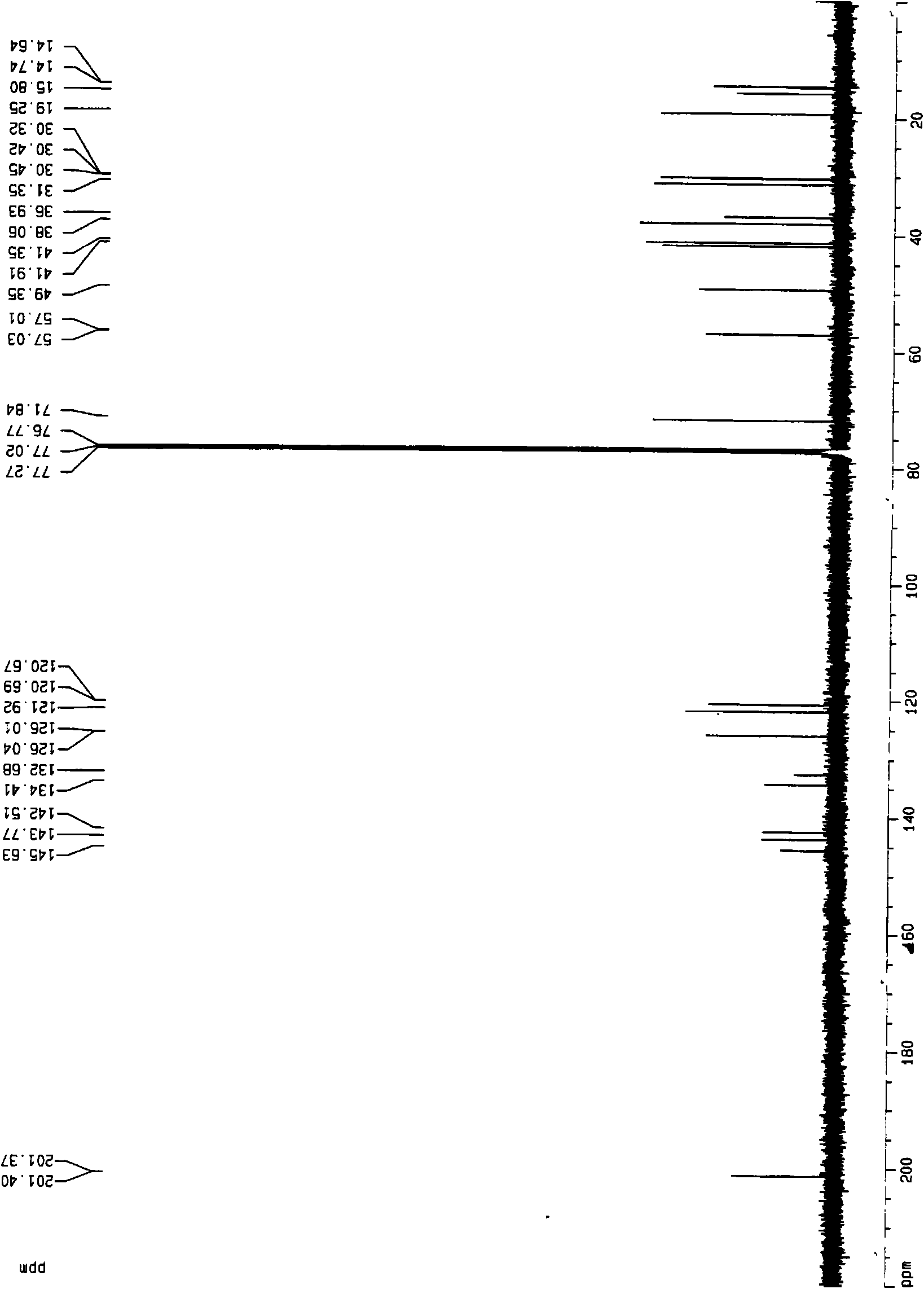
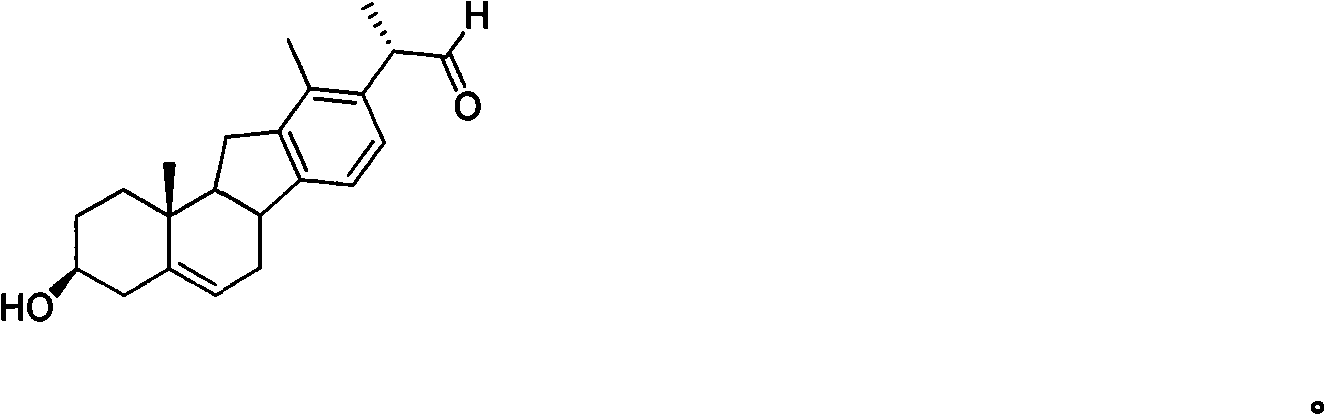
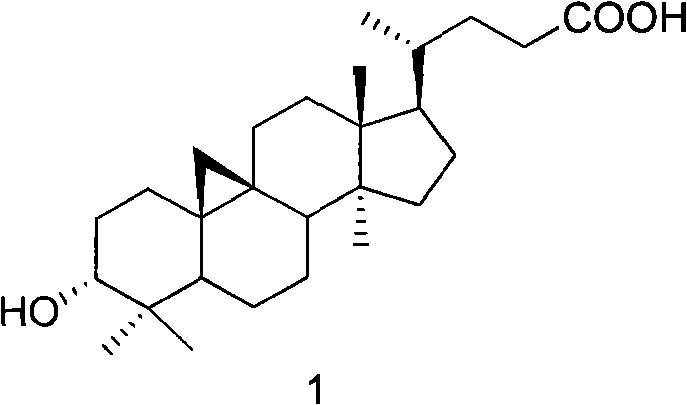
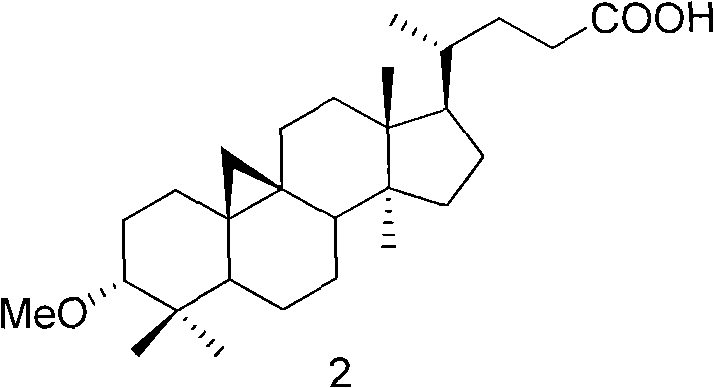
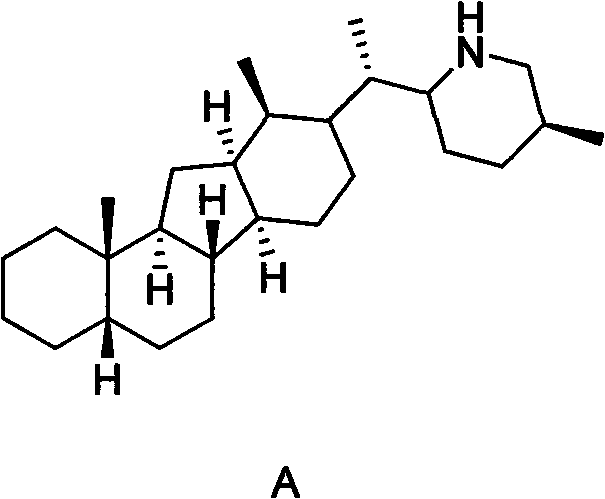
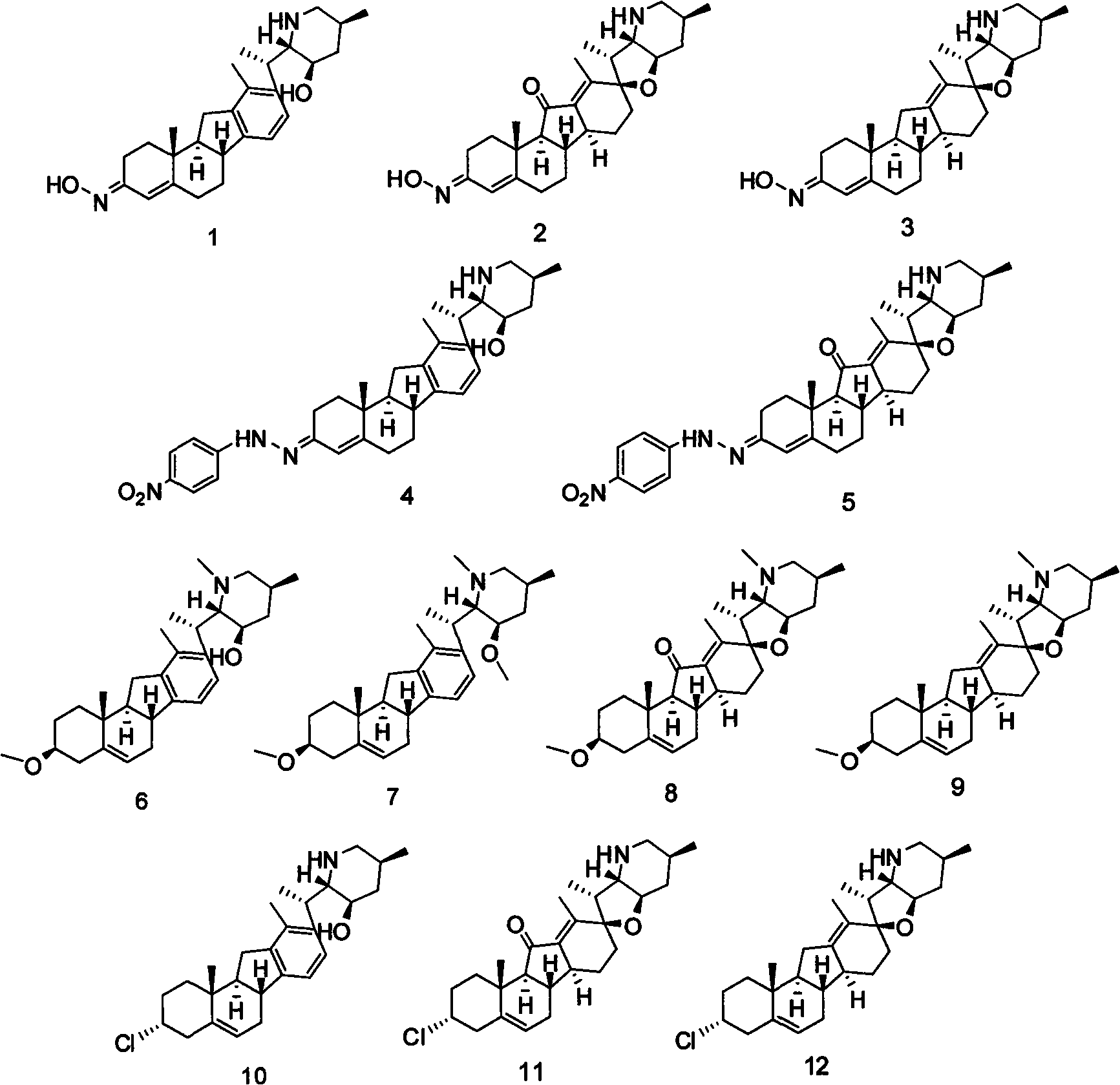
Veratramine degradation product veratrum fluorene aldehyde and the derivatives thereof, as well as the preparation and application thereof
InactiveCN101565446AEnhanced inhibitory effectOrganic active ingredientsSteroidsCancer cellSteroidal alkaloid
The invention provides a veratramine degradation product veratrum fluorene aldehyde 1 and derivatives thereof. The invention uses steroidal alkaloid veratramine (A) as raw material and prepares veratrum fluorene aldehyde by oxidative degradation of m-chloroperoxybenzoic acid to obtain 20 derivative compounds of the veratrum fluorene aldehyde 1 after further oxidation, reduction and condensation reaction. The 20 compounds of the invention have good inhibitory activity on cancer cells, wherein the compounds 1, 2, 5, 8, 13 and 17 have good inhibitory activity on cells of human pancreatic cancer cells BxPC-3 and SW1990, small-cell lung cancer cells NCI-H446, human colorectal cancer LOVO and the like, and the inhibitory activity of the compound veratrum fluorene aldehyde 1 is the most significant.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
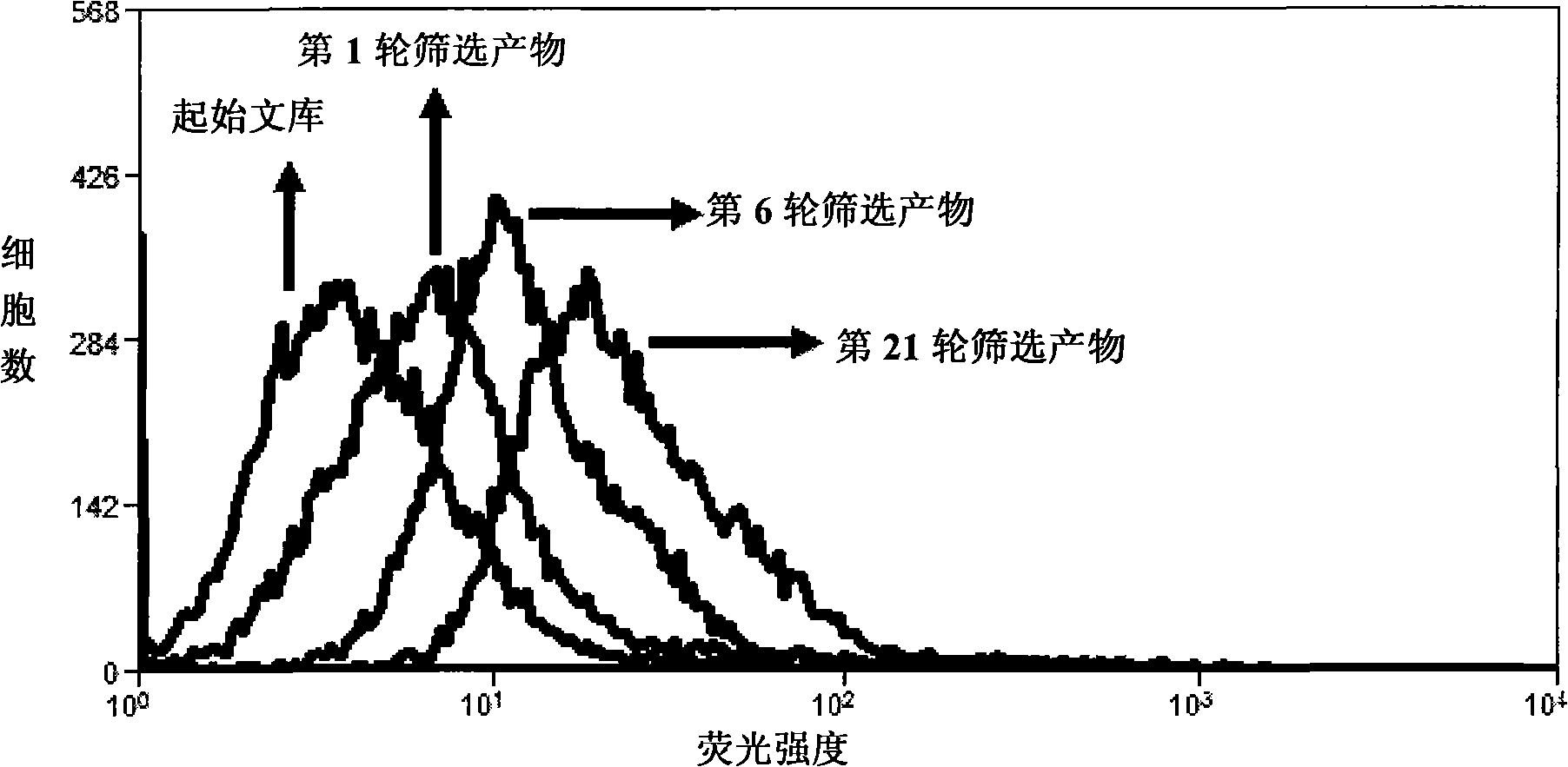
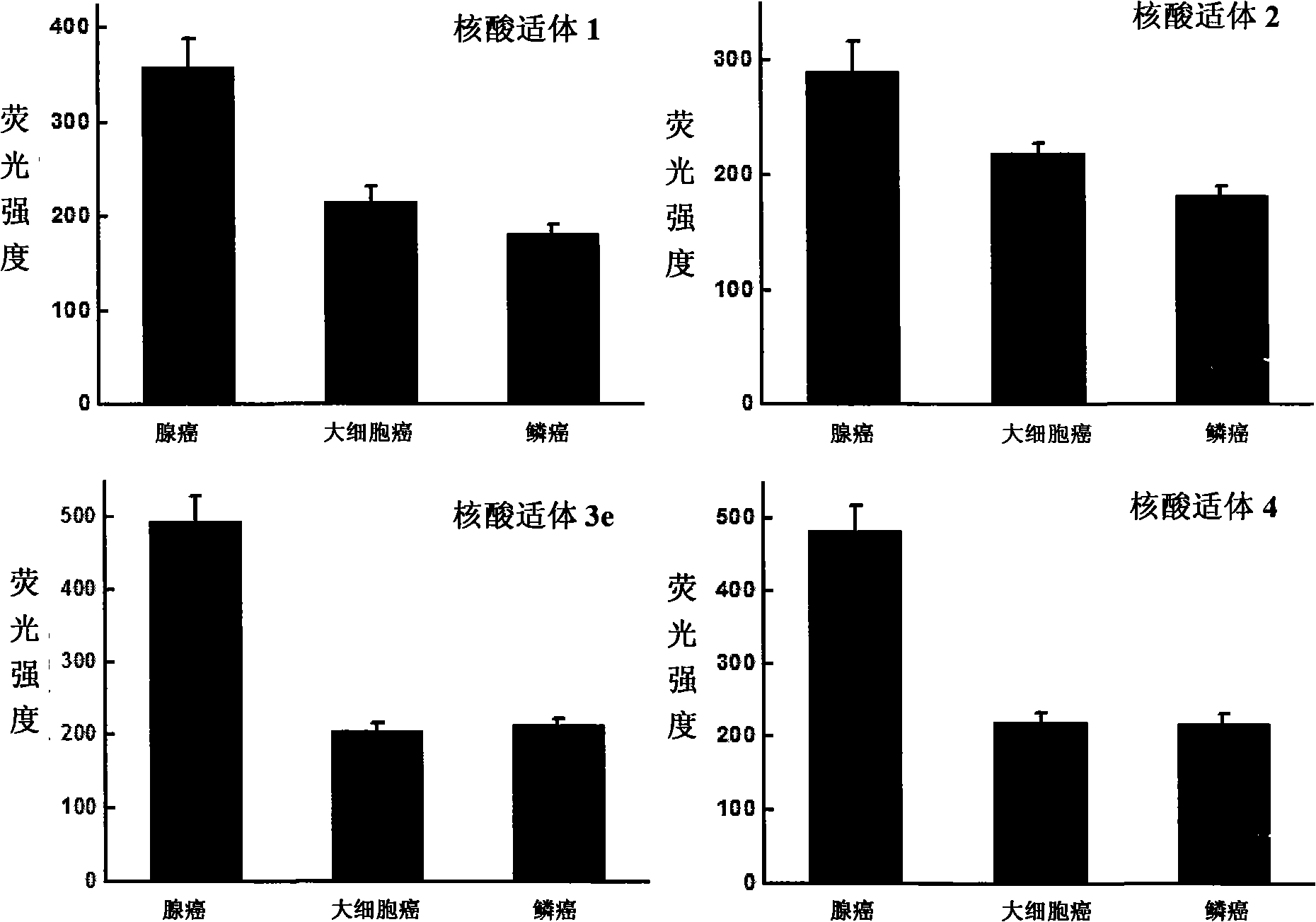
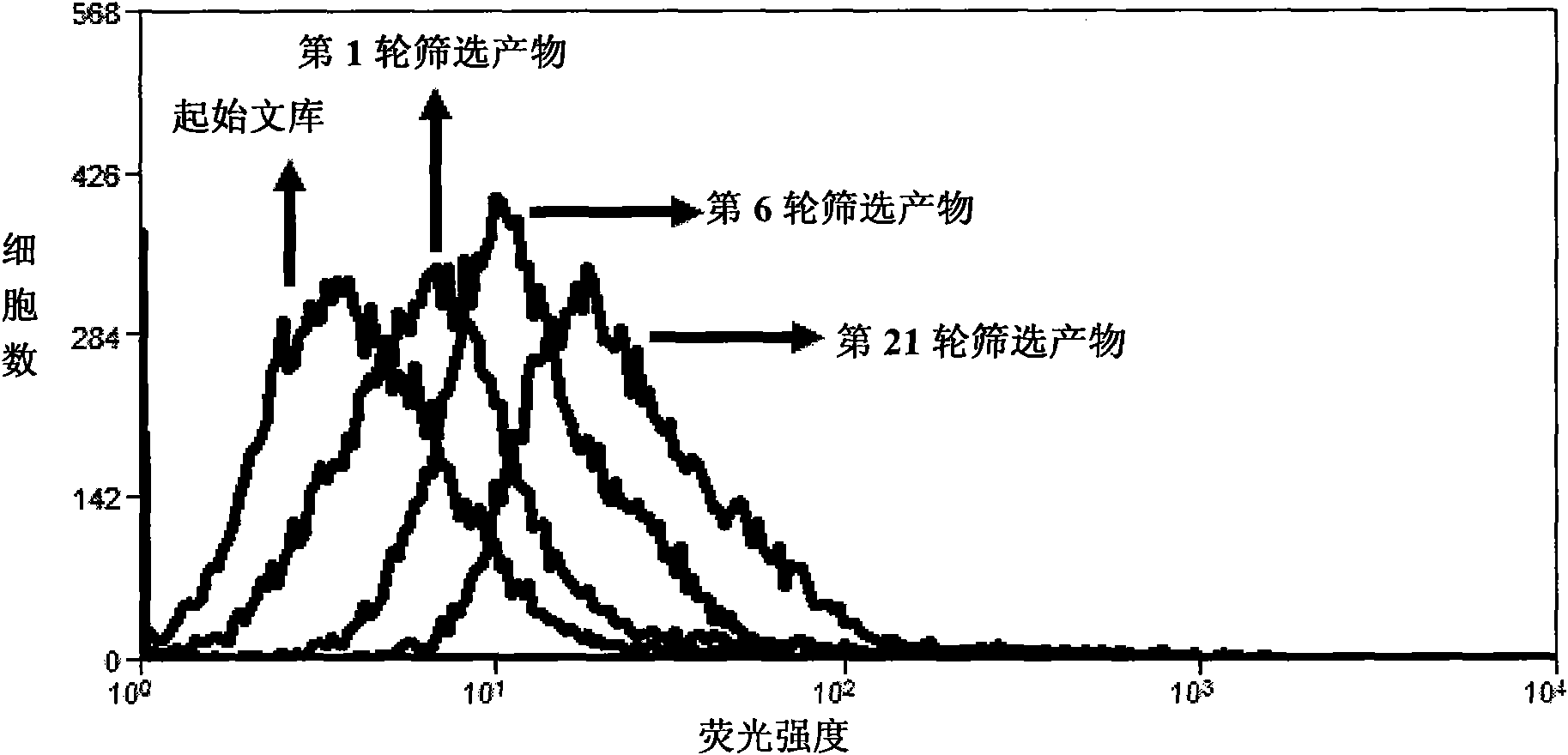
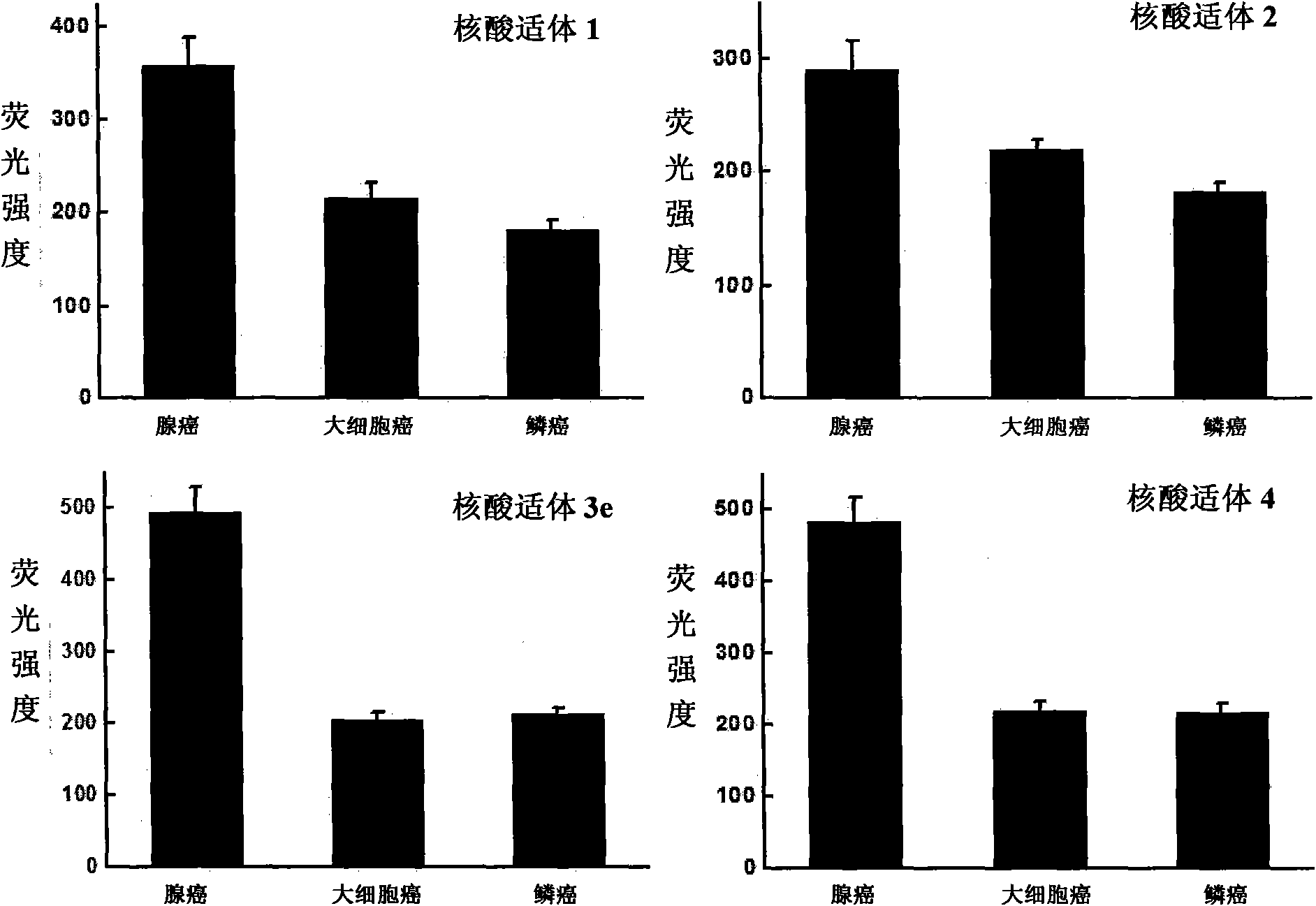
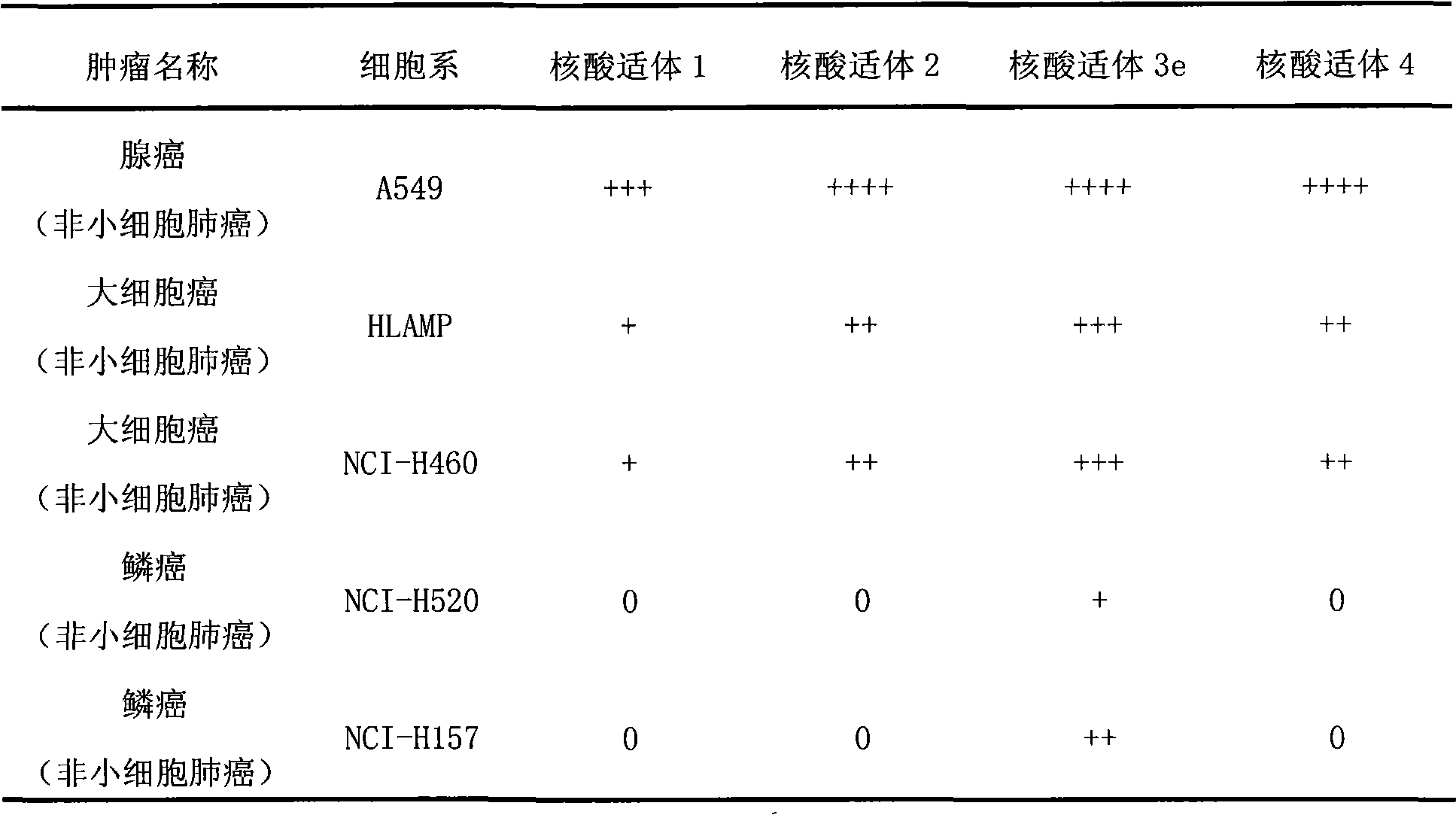
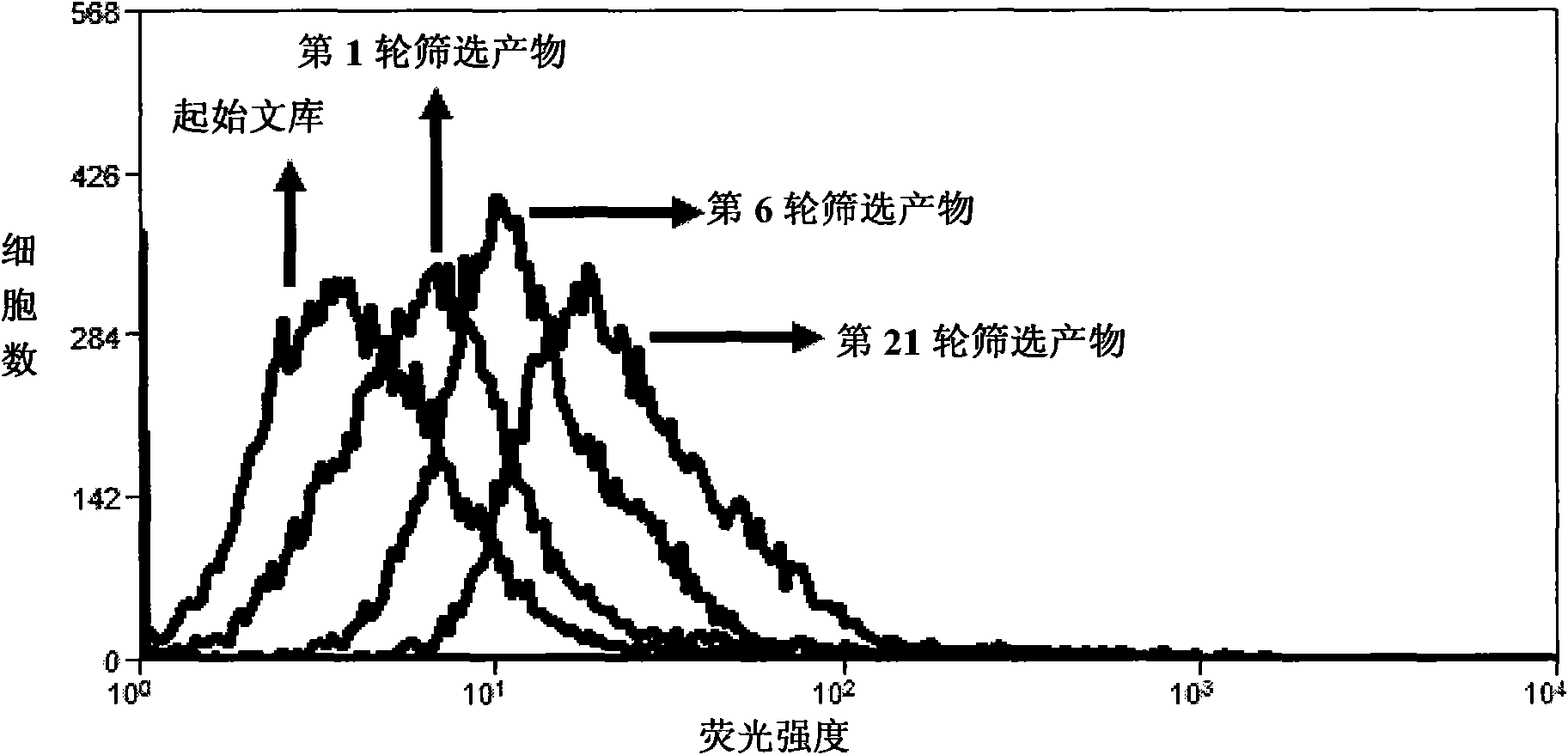
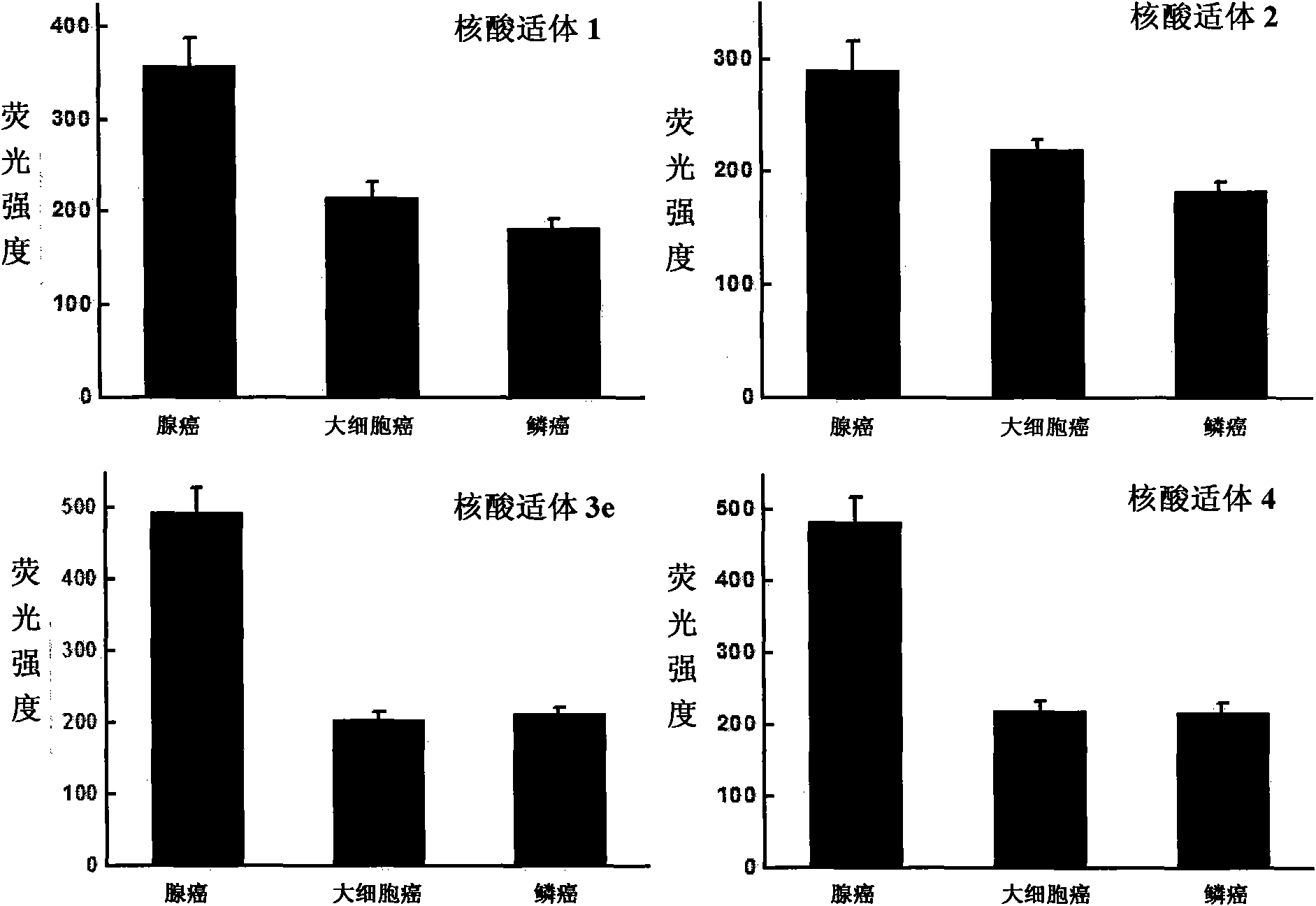
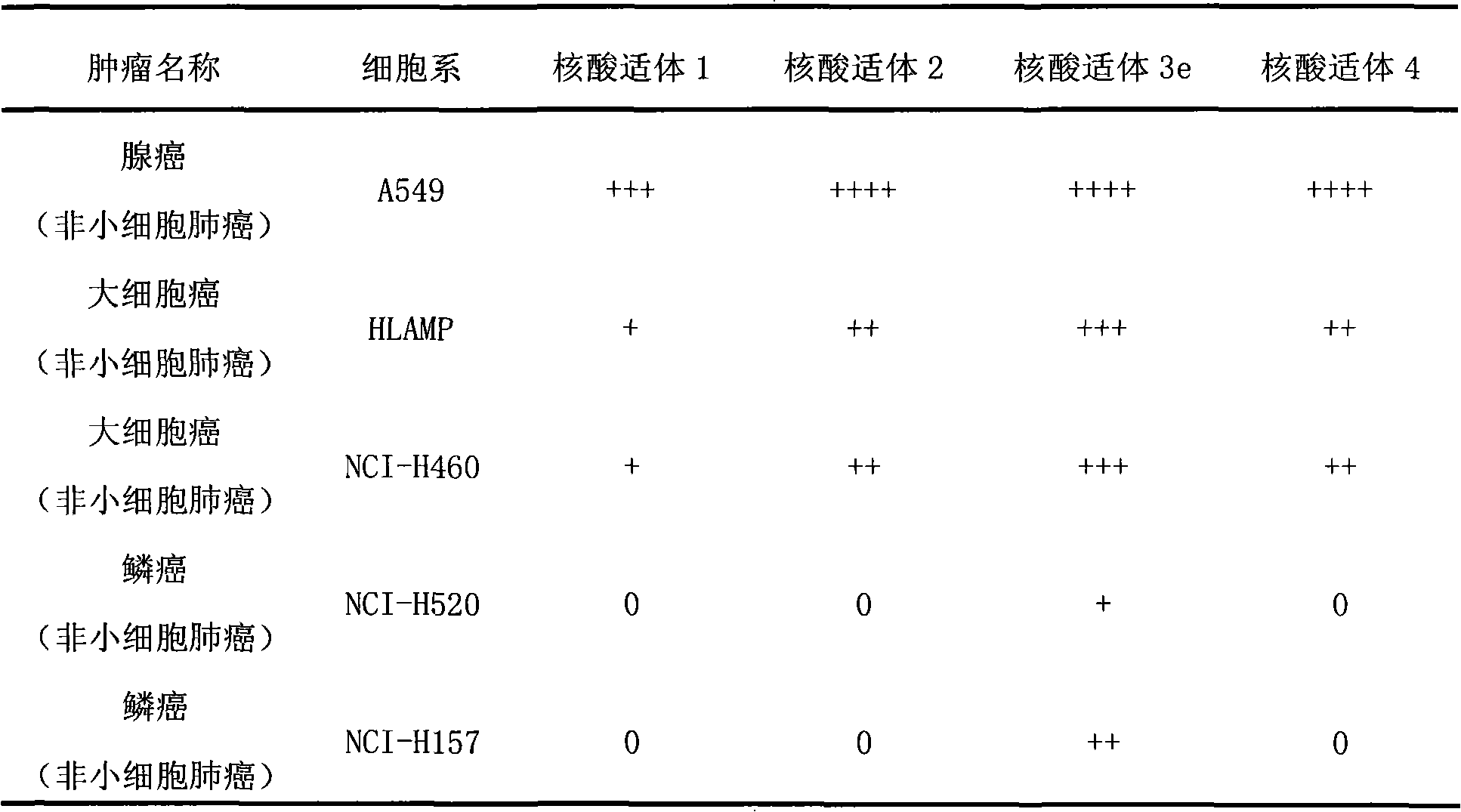
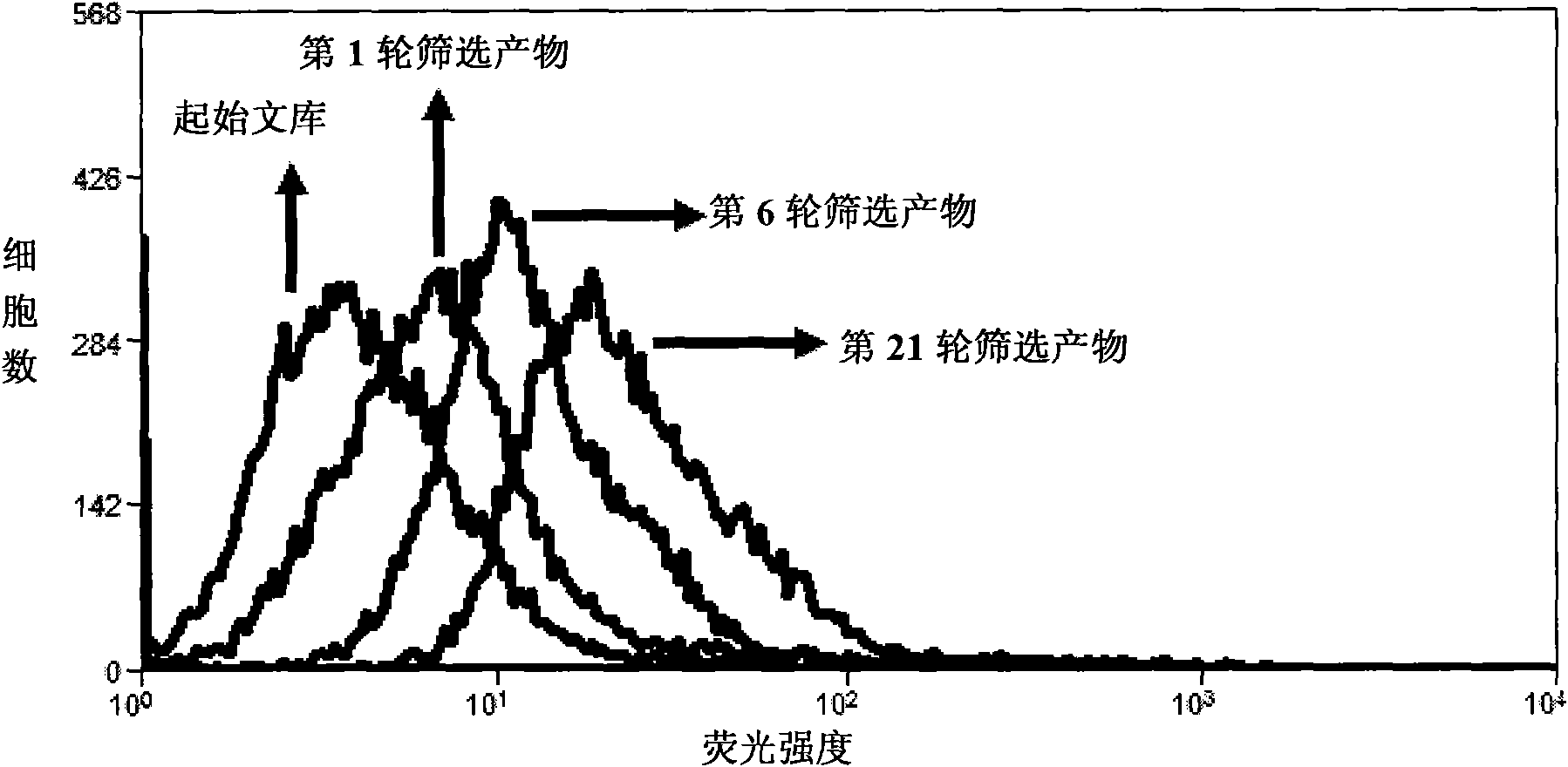
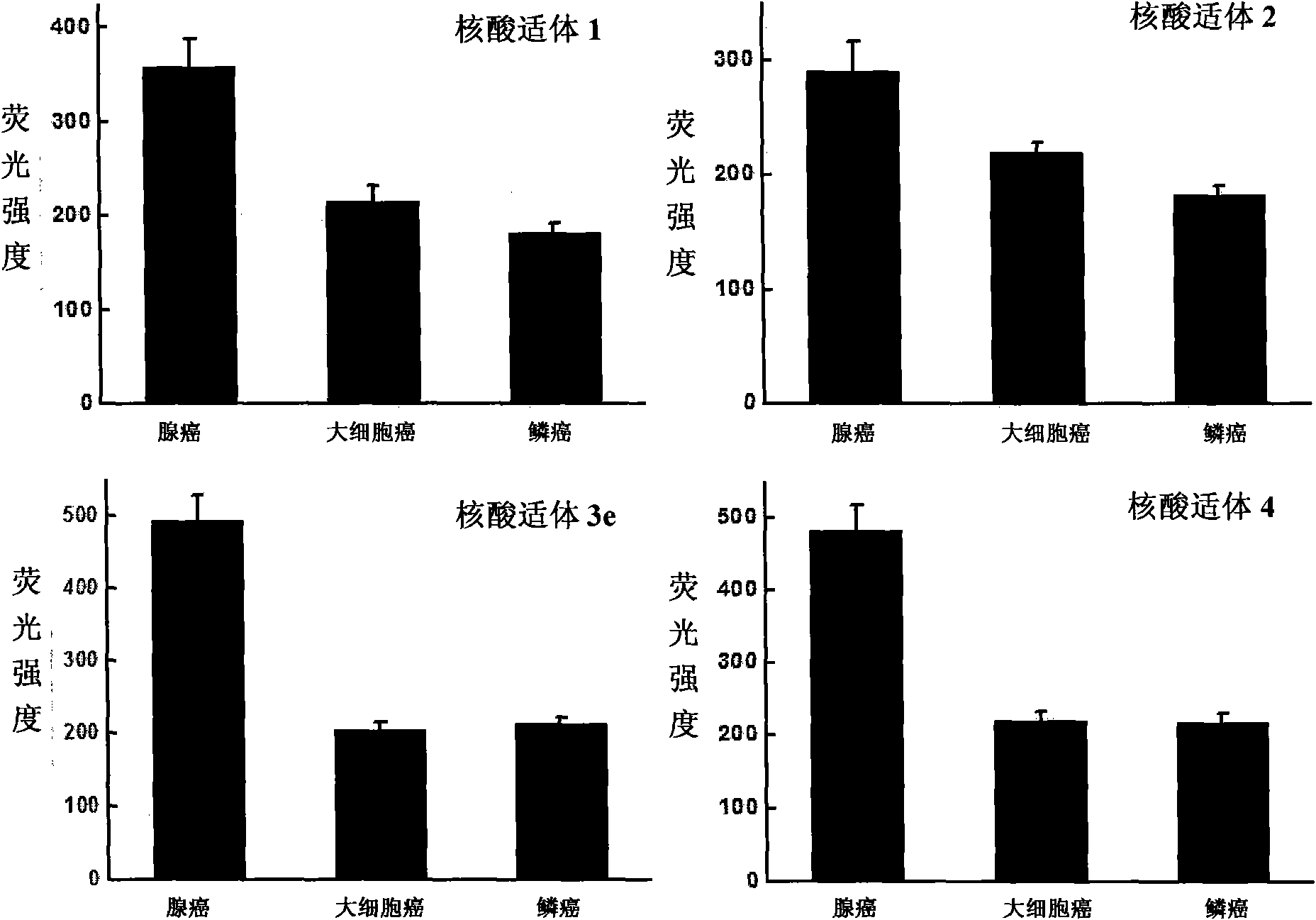
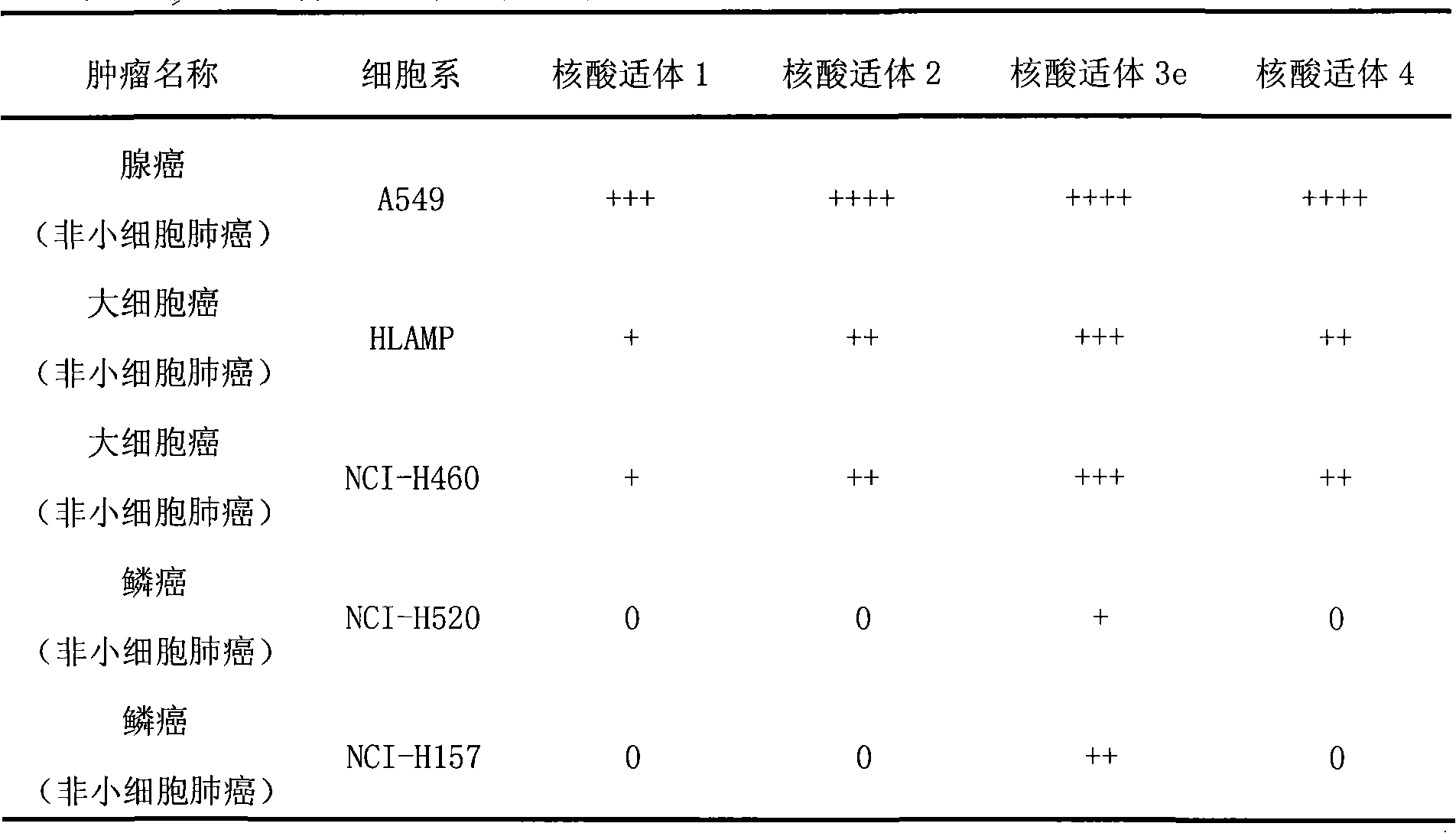
Aptamer for typing different subtypes of non-small cell lung cancer and method for screening the same
ActiveCN101538570AEarly diagnosisAccurate typingMicrobiological testing/measurementLibrary screeningTumor therapyScreening method
The invention discloses aptamer for typing different subtypes of non-small cell lung cancer and a method for screening the same. The aptamer provided by the invention is any DNA segment of nucleotide expressed by sequence 1 to sequence 9 in a sequence table. The aptamer is applied for typing different subtypes of non-small cell lung cancer. The aptamer of the invention can differentiate the different subtypes of non-small cell lung cancer on the molecule response signal under the condition of not knowing tumor markers of the non-small cell lung cancer. Using the aptamer of the invention for identifying the combined target is good for discovering the tumor markers of the different subtypes of non-small cell lung cancer, and earlier and exactly diagnosing and typing non-small cell lung cancer and discovering the tumor for treating new drug action targets.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Aptamer for grouping different subtype non-small cell lung cancers and screening method thereof
InactiveCN101955939AMicrobiological testing/measurementIndividual particle analysisAptamerScreening method
The invention discloses an aptamer for grouping different subtype non-small cell lung cancers and a screening method thereof. The aptamer provided by the invention is a DNA fragment having a nucleotide sequence represented by sequences from No.1 to No.2 in a sequence table. The aptamer can be applied to grouping of different subtype non-small cell lung cancers. The aptamer of the invention can group different subtypes of the non-small cell lung cancers according to a molecular response signal under the situation that a tumor marker of non-small cell lung cancer is unknown. The identification of a combination target of the aptamer by using the aptamer is favorable for finding the tumor marker of non-small cell lung cancer, for earlier diagnosis and more accurate grouping of the non-small cell lung cancers, and for finding a new medicinal function target of oncotherapy.
Owner:INST OF CHEM CHINESE ACAD OF SCI
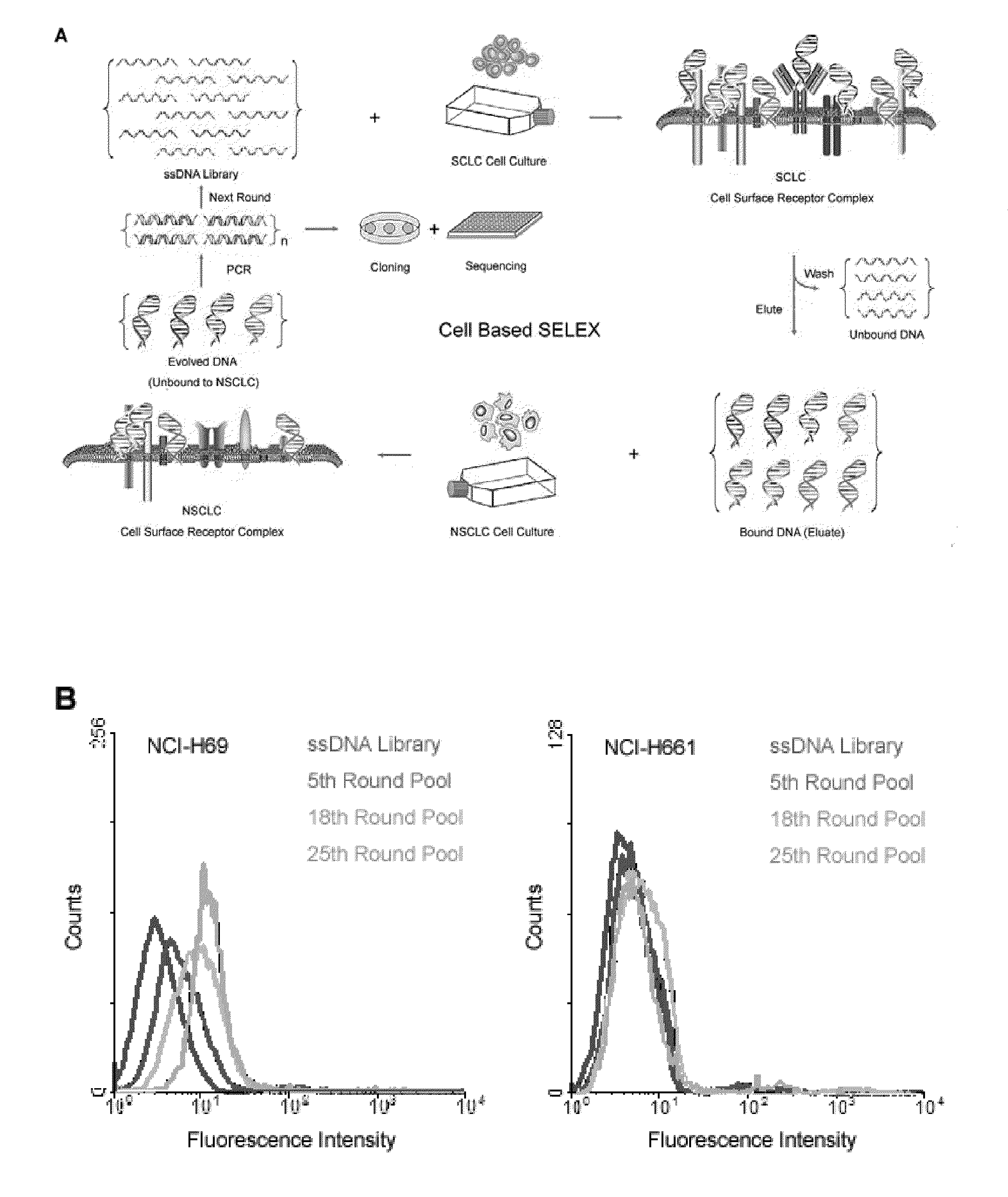
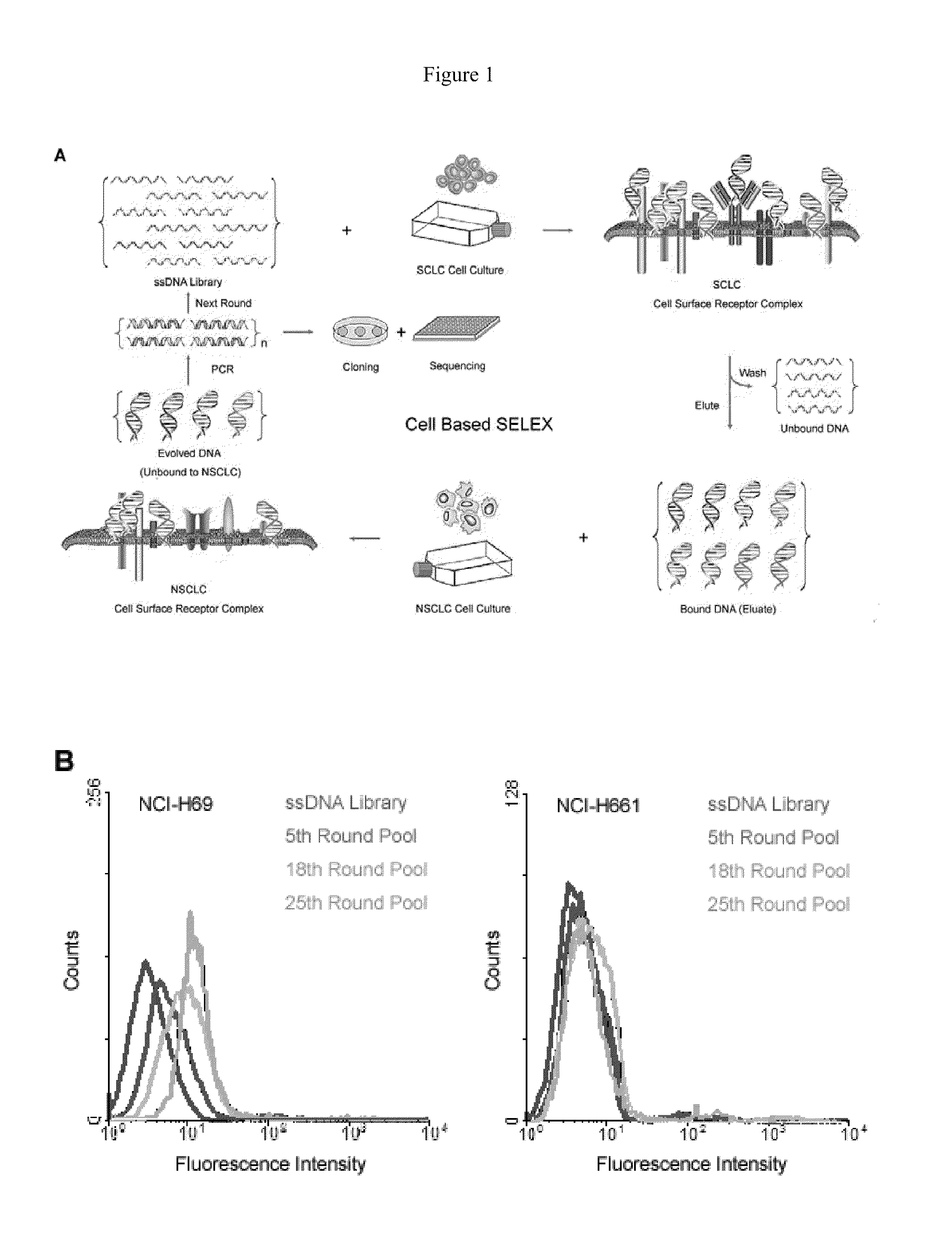
Aptamers that bind abnormal cells
InactiveUS20090239762A1High affinityStrong specificitySugar derivativesMicrobiological testing/measurementMixed cell cultureOn cells
A new aptamer approach for the recognition of specific small cell lung cancer (SCLC) cell surface molecular markers relies on cell-based systematic evolution of ligands by exponential enrichment (cell-SELEX) to evolve aptamers for whole live cells that express a variety of surface markers representing molecular differences among cancer cells. When applied to different lung cancer cells including those from patient samples, these aptamers bind to SCLC cells with high affinity and specificity in different assay formats. When conjugated with magnetic and fluorescent nanoparticles, the aptamer nano-conjugates could effectively extract SCLC cells from mixed cell media for isolation, enrichment, and sensitive detection.
Owner:TAN WEIHONG +1
Human non-small cell lung cancer cell line, and establishment method and application thereof
PendingCN104694476ARandom combinationBest practiceMicrobiological testing/measurementMicroorganism based processesCancer cellOncology
The invention discloses a non-small cell lung cancer cell line, and an establishment method and application thereof. The human non-small cell lung cancer cell line is preserved in China Center for Type Culture Collection with an accession number of CCTCC NO: C2012128. The establishment method comprises the following steps: step 1, acquiring a fresh clinical human non-small cell lung cancer surgical specimen, cutting the specimen into small blocks with a size of 20 to 50 mg and inoculation mammals with the block through subcutaneous puncture; and step 2, in 70 to 90 days after the puncture-inoculation, killing a tumor-bearing animal, taking tumor tissue out of the tumor-bearing animal and carrying out primary culture and subculture of cancer cells. According to the invention, the human non-small cell lung cancer cell line has the advantages of stable properties, capability of realizing stable multiple passage, a high tumor formation rate, a short latent period and good homogeneity; meanwhile, the human non-small cell lung cancer cell line has acquired drug resistance to gefitinib, can be used to analyze correlation between in-vitro and in-vivo drug sensibilities and drug resistance and is the ideal cell line for basic research on and preclinical application of human non-small cell lung cancer.
Owner:SHANGHAI CHEMPARTNER CO LTD +1
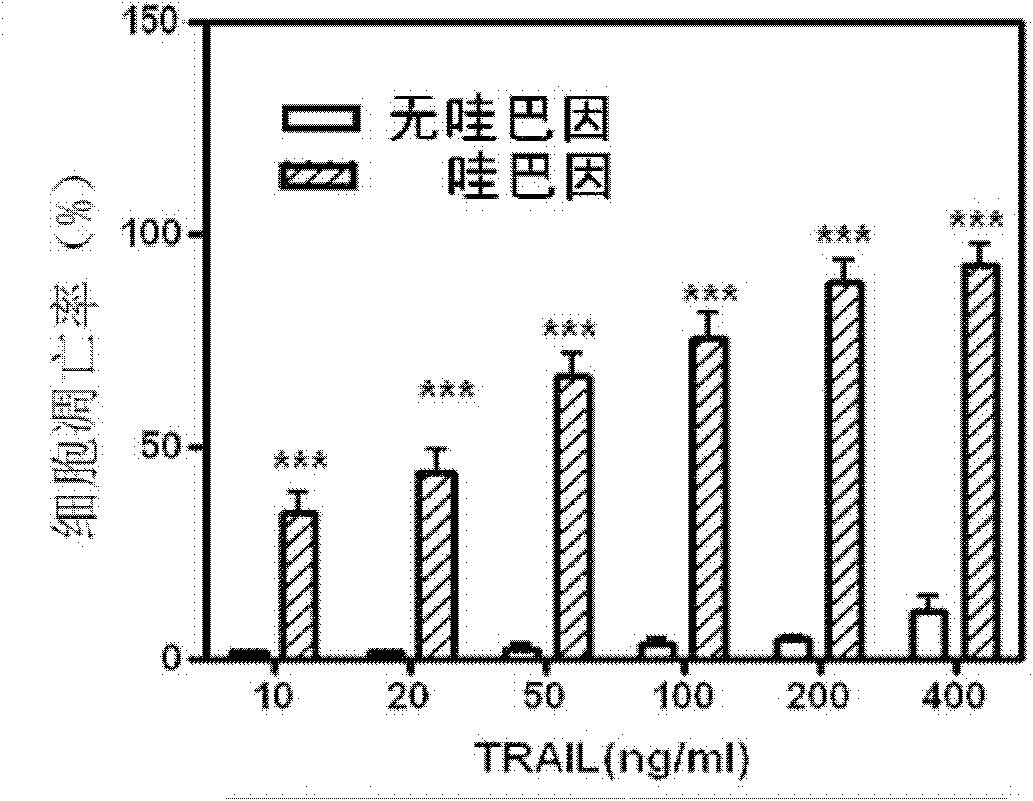
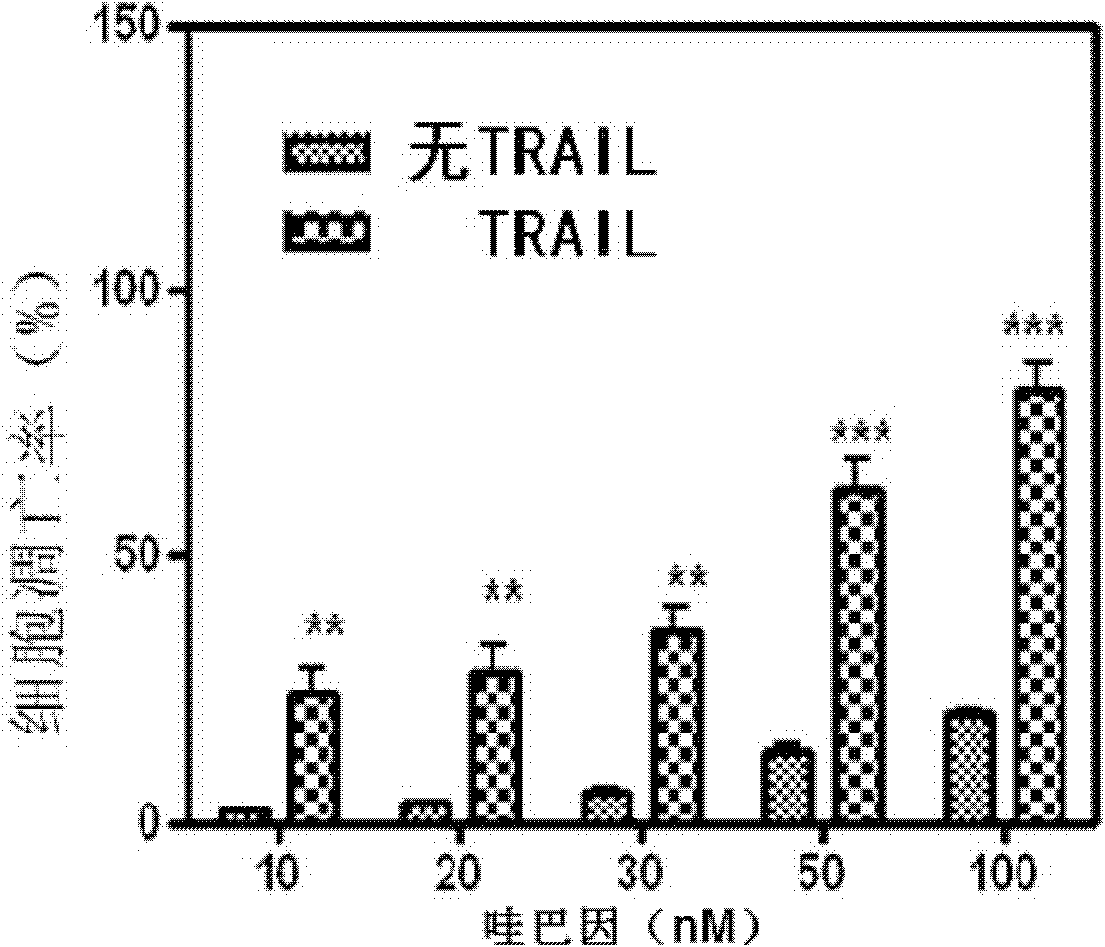
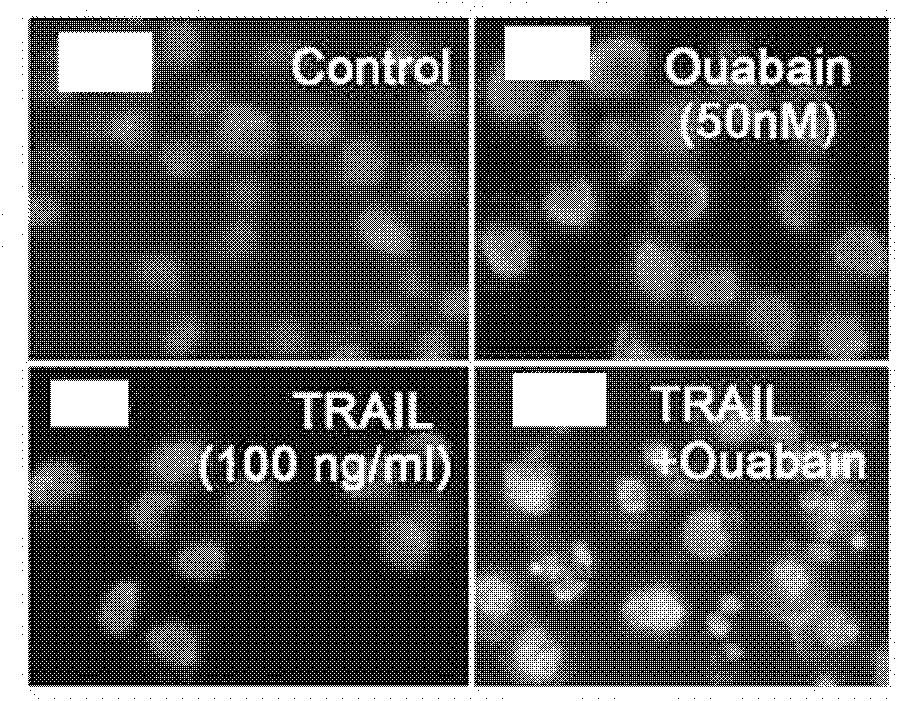
Application of uabain for enhancing cellular sensitivity of non-small cell lung cancer (NSCLC)
The invention relates to a new application of sodium-potassium ATP enzyme inhibitor uabain for reversing tolerance of cells of non-small cell lung cancer (NSCLC) to targeted anti-tumor drugs. Particularly, the uabain can be used as a sensitizer for treating the NSCLC and is combined with TRAIL to be used for treating the NSCLC.
Owner:NANJING UNIV
Microtubule inhibitor as well as preparation method and application thereof
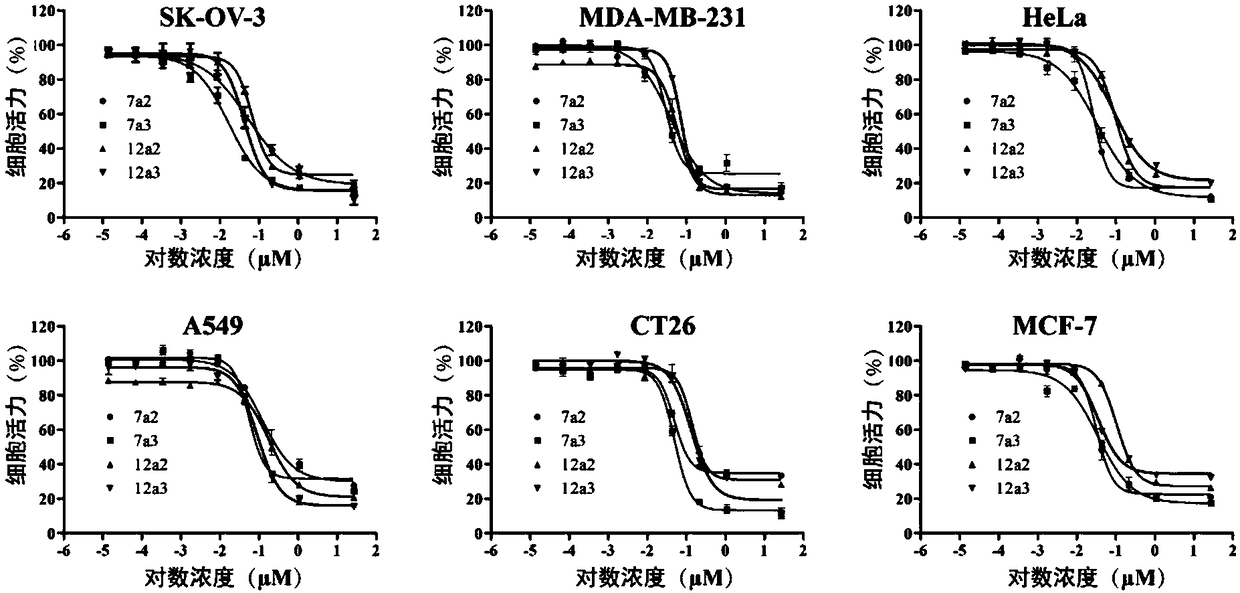
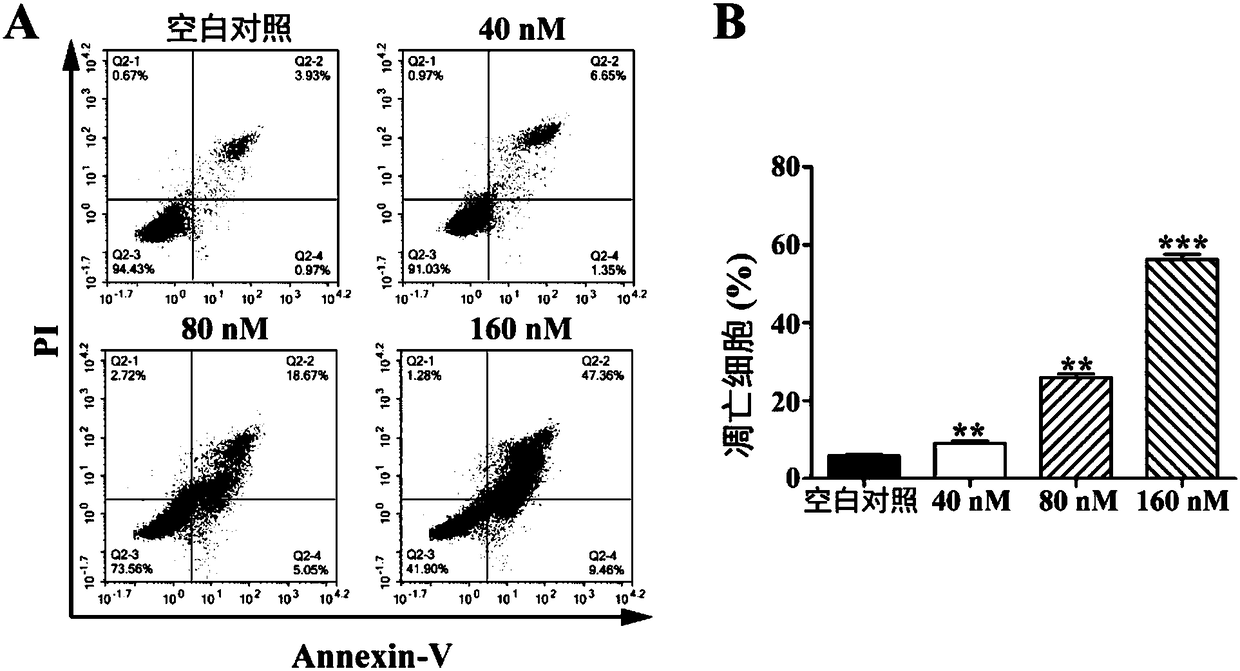
The invention relates to a microtubule inhibitor as well as a preparation method and application thereof, belongs to the field of chemical medicines and aims to solve the problem that the biological activity is rapidly reduced because the existing microtubule inhibitor CA-4 cisoid conformation is easily transformed into trans-conformation. The technical scheme provides a compound expressed by a formula I as well as pharmaceutically acceptable salts or crystals thereof. Experiment results show that the compound shows extremely strong in-vitro anti-proliferation activity in tumor cells such as ovarian cancer cells, cervical cancer cells, non-small cell lung cancer cells, colon cancer cells and breast cancer cells, can obviously inhibit the tumor progression in the body, and is high in safety; the formula I is as shown in the description.
Owner:WEST VAC BIOPHARMA CO LTD
Aziridinyl quinone antitumor agents based on indoles and cyclopent[b]indoles
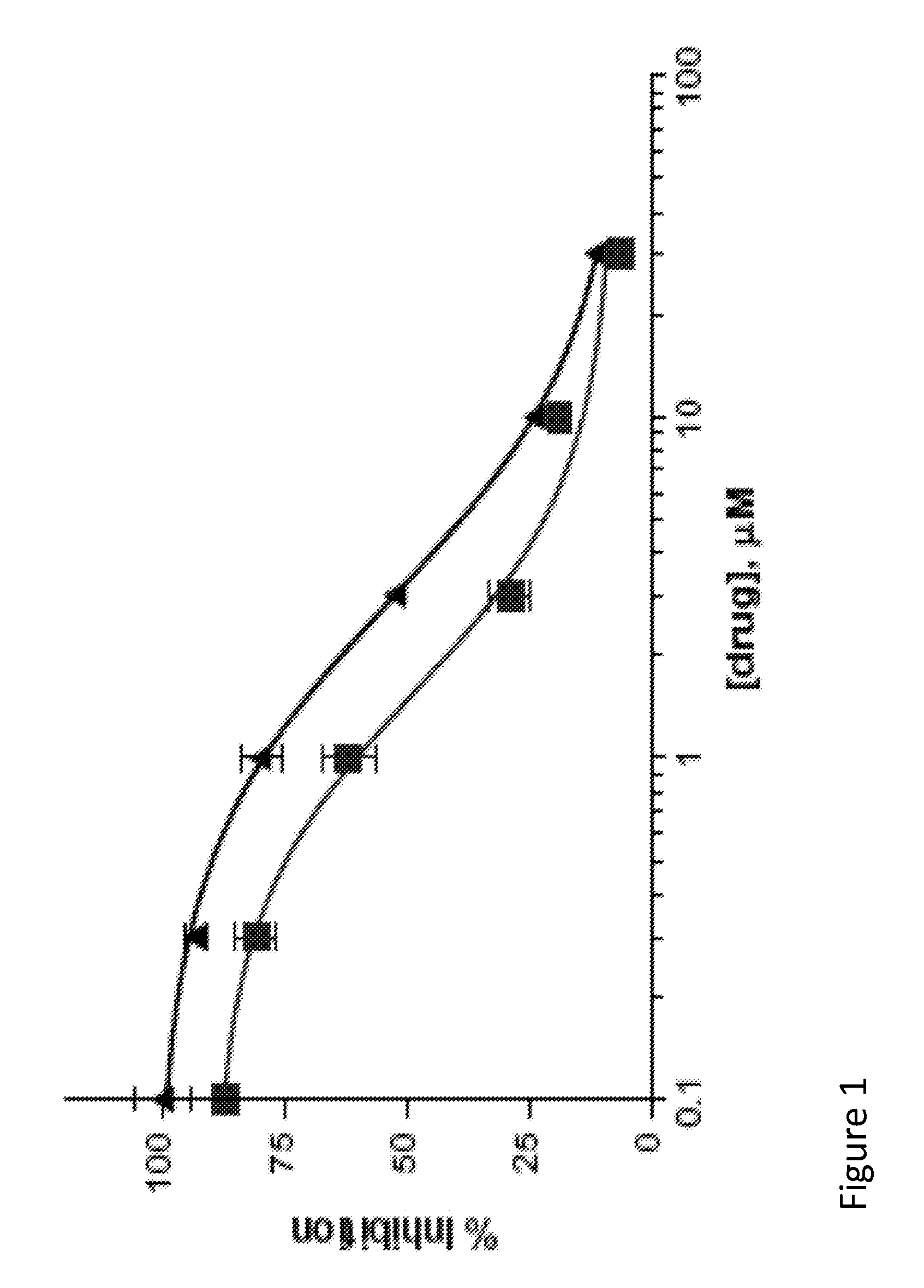
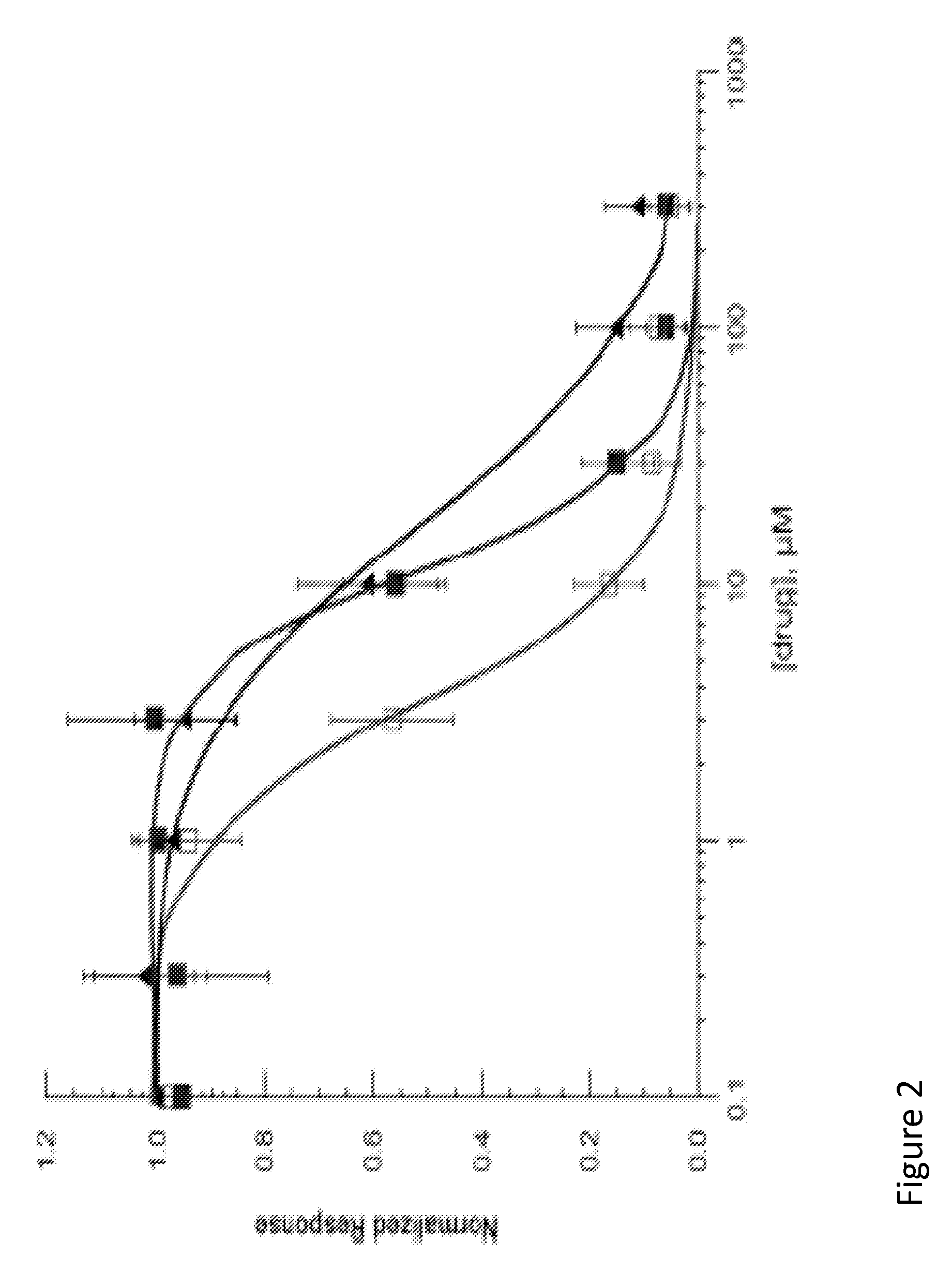
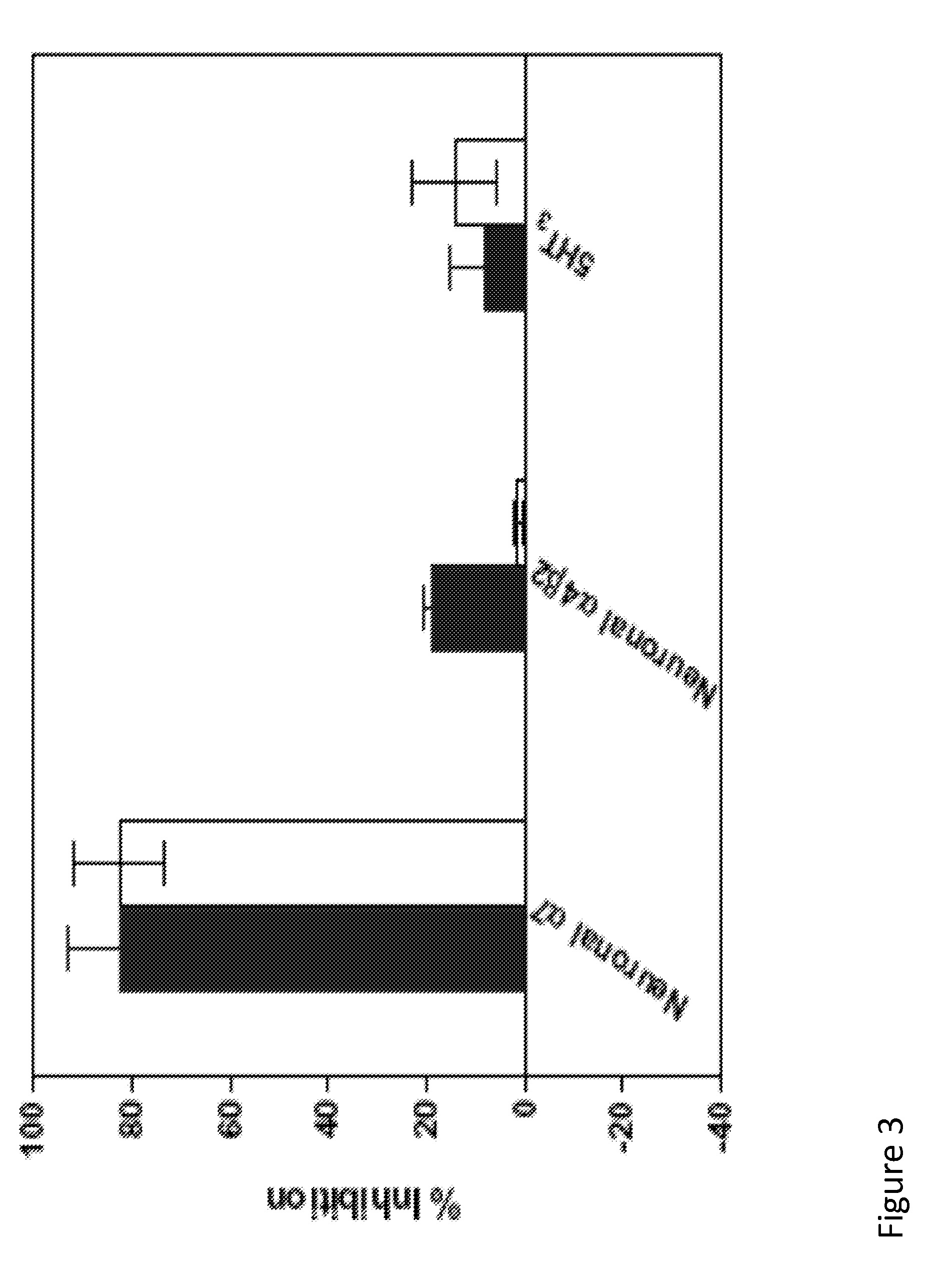
A large number of aziridinyl quinones represented by Series 1-9 were studied with respect to their DT-diaphorase substrate activity, DNA reductive alkylation, cytostatic / cytotoxic activity, and in vivo activity. As a result generalizations have been made with respect with respect to the following: DT-diaphorase substrate design, DT-diaphorase-cytotoxicity QSAR, and DNA reductive alkylating agent design. A saturating relationship exists between the substrate specificity for human recombinant DT-diaphorase and the cytotoxicity in the human H460 non-small-cell lung cancer cell line. The interpretation of this relationship is that reductive activation is no longer rate limiting for substrates with high DT-diaphorase substrate specificities. High DT-diaphorase substrate specificity is not desirable in the indole and cylopent[b]indole systems because of the result is the loss of cancer selectivity along with increased toxicity. We conclude that aziridinyl quinones of this type should possess a substrate specificity (VMAX / KM )<10x10-4 s-1 for DT-diaphorase in order not to be too toxic or nonselective. While some DNA alkylation was required for cytostatic and cytotoxic activity by Series 1-9, too much alkylation results in loss of cancer selectivity as well as increased in vivo toxicity. Indeed, the most lethal compounds are the indole systems with a leaving group in the 3a-position (like the antitumor agent EO-9). We conclude that relatively poor DNA alkylating agents (according to our assay) show the lowest toxicity with the highest antitumor activity.
Owner:ARIZONA STATE UNIVERSITY
Abies holophylla triterpenoid compound, extraction separation thereof and application thereof
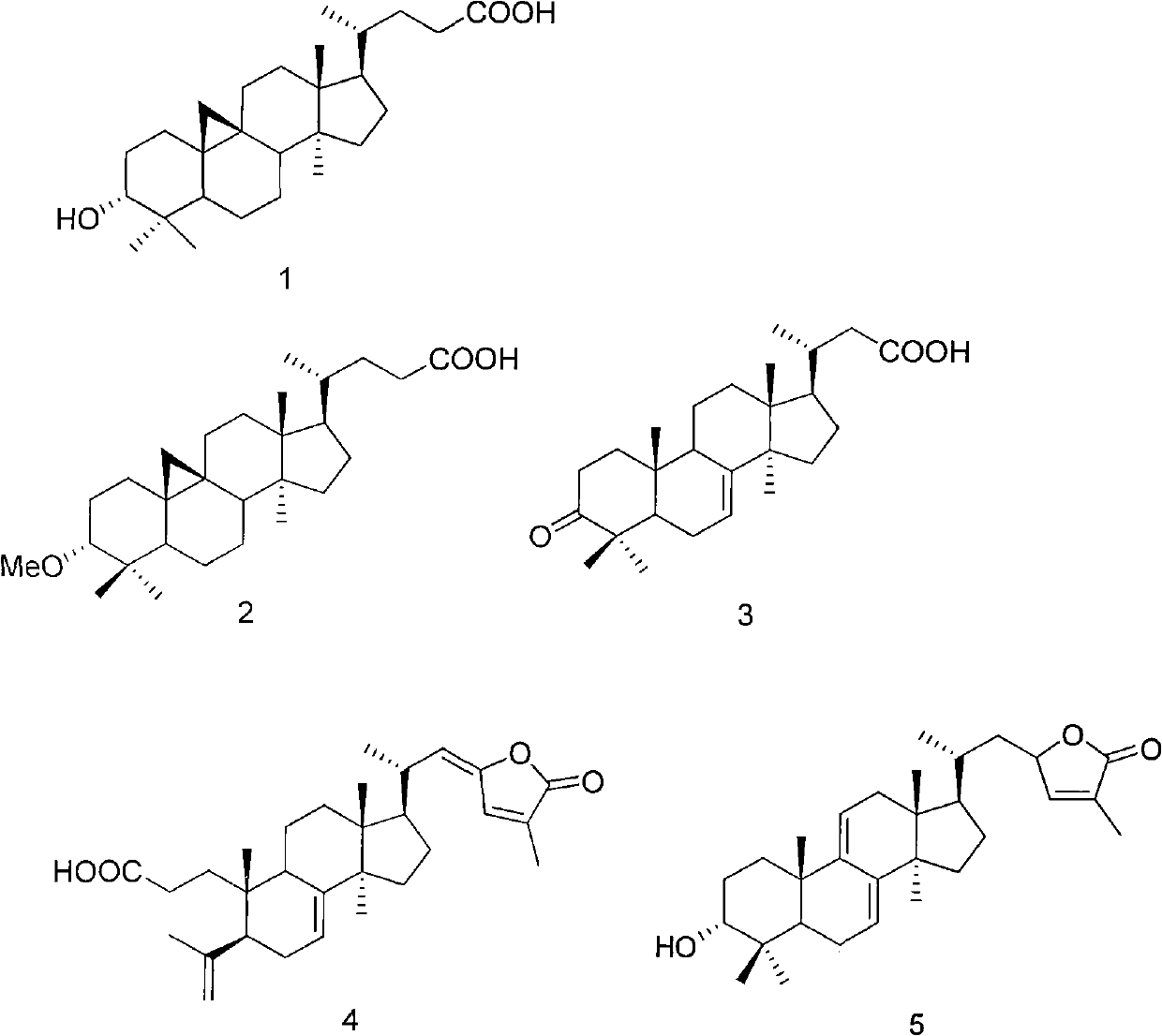
InactiveCN102030800ALittle toxic effectGood curative effectOrganic active ingredientsSteroidsSide effectBULK ACTIVE INGREDIENT
The invention provides an abies holophylla triterpenoid compound, which comprises the following compounds 1, 2, 3, 4 and 5. The compounds are subjected to an experiment of cytotoxic activity, and a result shows that the five compounds have good inhibitory activity and excellent anti-tumor effect on human lung cancer cells, human intestinal cancer cells, human small cell lung cancer cells and human breast cancer cells. The five compounds are proved by a curative effect experiment on sarcoma (entities) of mice S180 to have the obvious tumor-inhibitory effect. The abies holophylla triterpenoid compound can be used for preparing anti-cancer medicaments. The medicaments of the invention consist of the abies holophylla triterpenoid compound serving as active ingredients and a conventional medicinal carrier, and can be prepared into preparations according to the conventional method. The medicaments of the invention are Chinese medicinal preparations, have small toxic and side effect and highcurative effect, provide new anti-cancer medicaments clinically, have large practical application value and can create good social benefit.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Aptamer for typing different non-small cell lung cancer subtypes and screening method thereof
InactiveCN101914542AEarly diagnosisAccurate typingMicrobiological testing/measurementIndividual particle analysisNon small cell cancerTumor therapy
The invention discloses an aptamer for typing different non-small cell lung cancer subtypes and a screening method thereof. The aptamer provided by the invention is a DNA segment containing a nucleotide shown as any one of formulae 1 to 9 in a sequence table, can be applied to the cell typing of the different non-small cell lung cancer subtypes, can realize the distinguishing of the different non-small cell lung cancer subtypes by molecule response signals under the condition that tumor markers of non-small cell lung cancer are unknown, and is combined with targets for identification so as tobe favorable for discovering the tumor markers of the different non-small cell lung cancer subtypes, earlier diagnosing and more accurately typing the non-small cell cancer and discovering novel medicament functional targets for tumor treatment.
Owner:INST OF CHEM CHINESE ACAD OF SCI
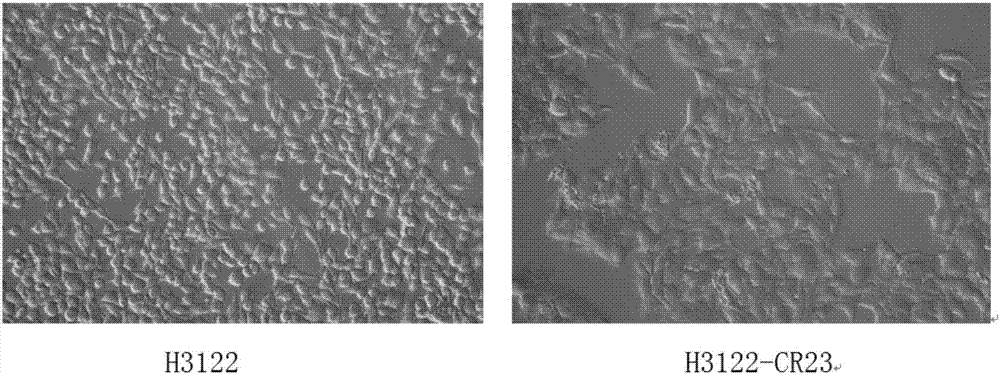
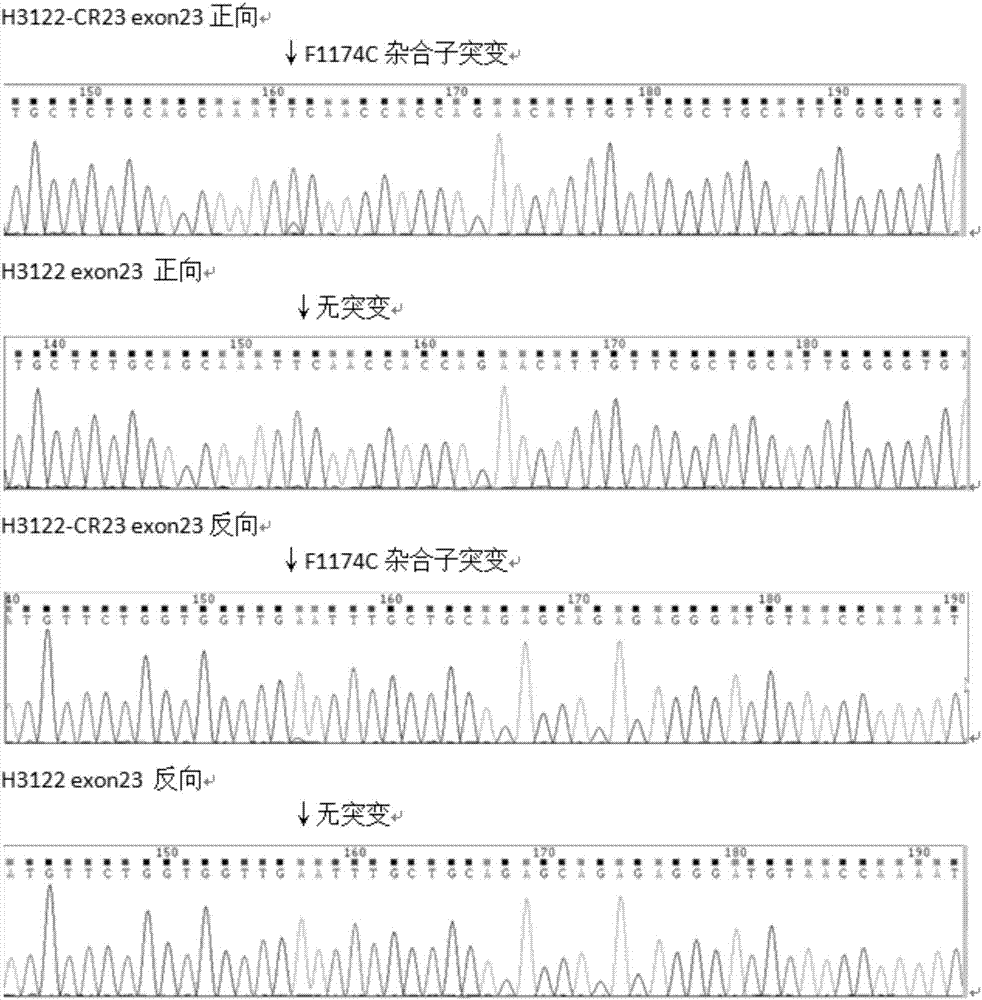
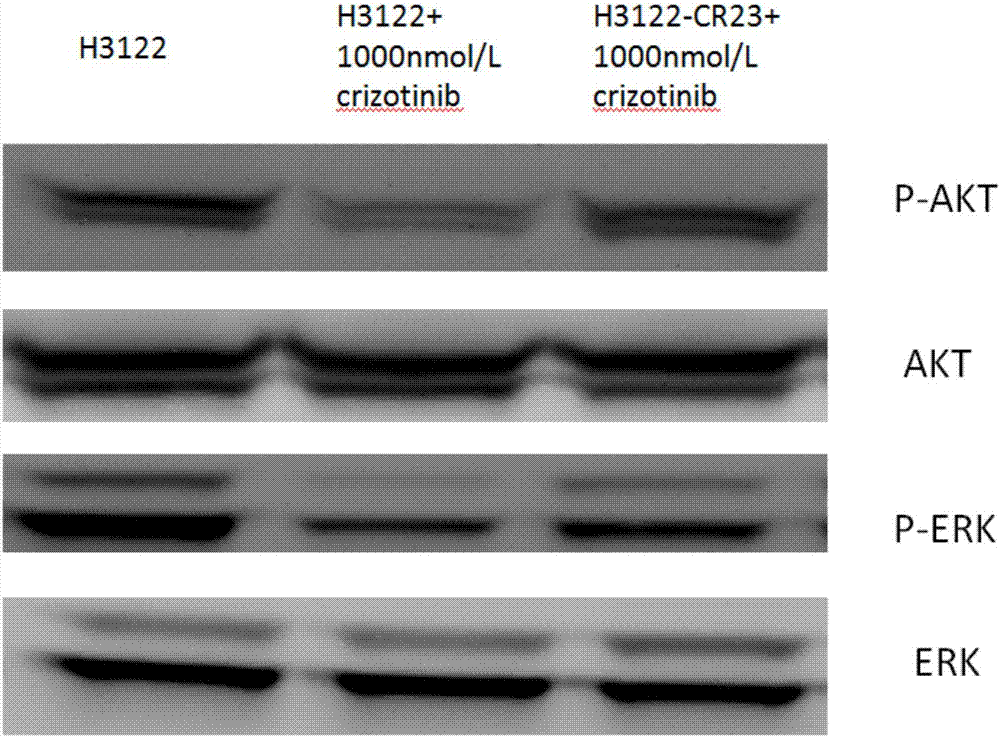
Anti-crizotinib human non-small cell lung cancer cell strain H3122-CR23 and application thereof
ActiveCN107267458AIncrease production capacityMicrobiological testing/measurementMammal material medical ingredientsSocial benefitsSmall cell lung carcinoma cell
The invention discloses a novel anti-crizotinib human non-small cell lung cancer cell strain which is named as H3122-CR23 and has an accession number of CCTCC No. C201780. The cell strain has F1174C mutation in an ALK kinase area. The cell strain H3122-CR23 is applicable to research on the morphological and biological characteristics of human anti-crizotinib non-small cell lung cancer cells, research on the drug resistance mechanisms of tumors, development of drugs for drug resistance reversal of tumors, analysis of the susceptibility of antitumor drugs, screening and assessment of antitumor drugs, research on more efficient tumor treatment methods, etc., has high application value in scientific research and production, and is expected to produce good scientific research, economic and social benefits.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Jervine steroid alkaloid derivatives and preparation and application thereof
The invention provides jervine steroid alkaloid derivatives. Steroidal alkaloids, namely veratramine, jervine and cyclopamine are taken as raw materials, and subjected to chemical modification to obtain ninety-three derivatives, which comprise N-hydrocarbon and N-acylate. In the invention, cell lines such as human pancreatic cancer cells B*PC-3 and SW1990, a small cell lung cancer cell NCI-H466, a non-small cell lung cancer cell NCI-H157 and the like are selected, the antitumor activity of all compounds are evaluated by an MTT method, and results show that the derivatives 41, 43 and 58 have obvious antitumor activity, the other eight derivatives 10, 18, 19, 38, 45, 48, 50 and 92 also show medium antitumor activity, and the derivatives can be used for preparing antitumor medicaments.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
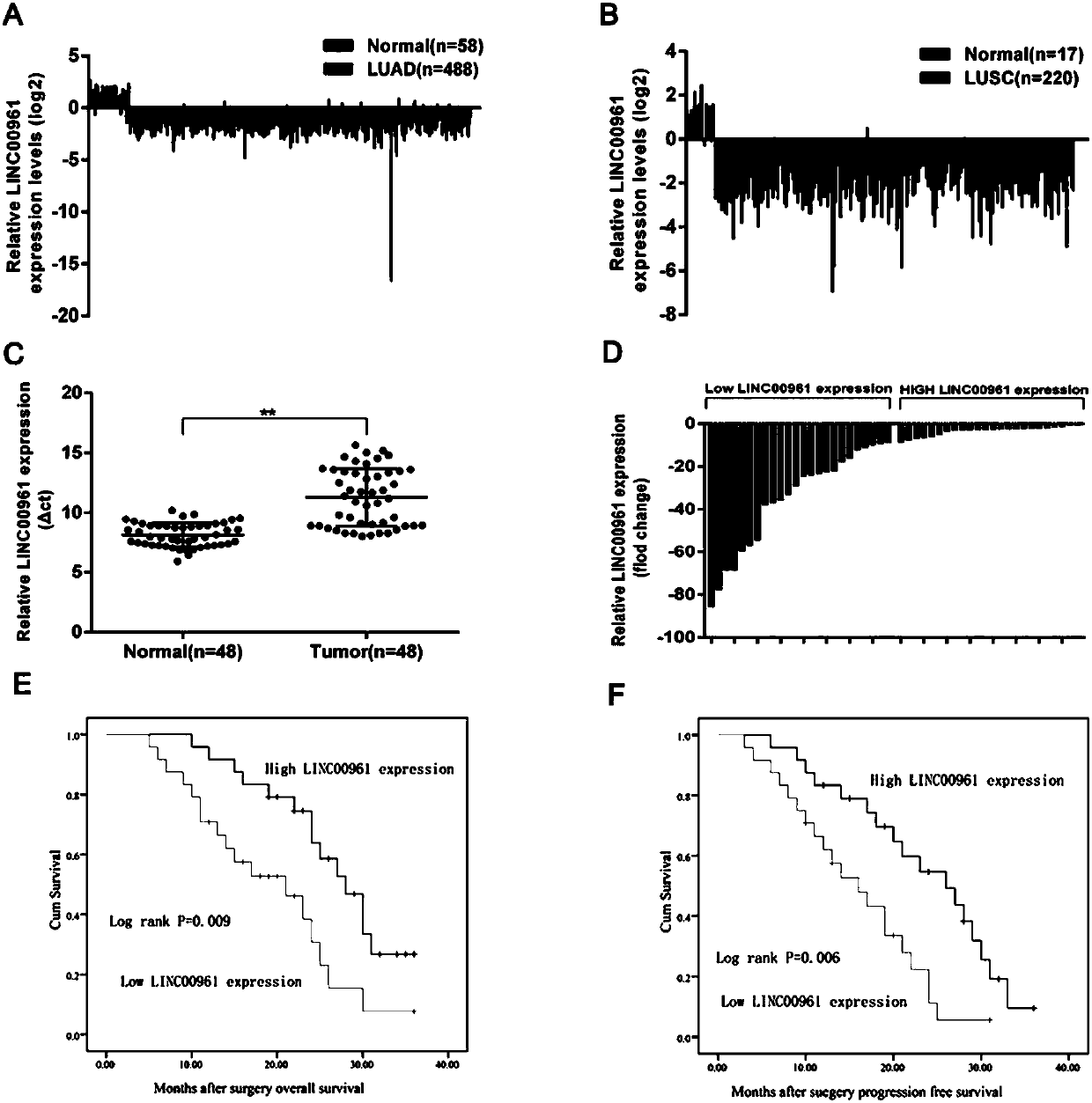
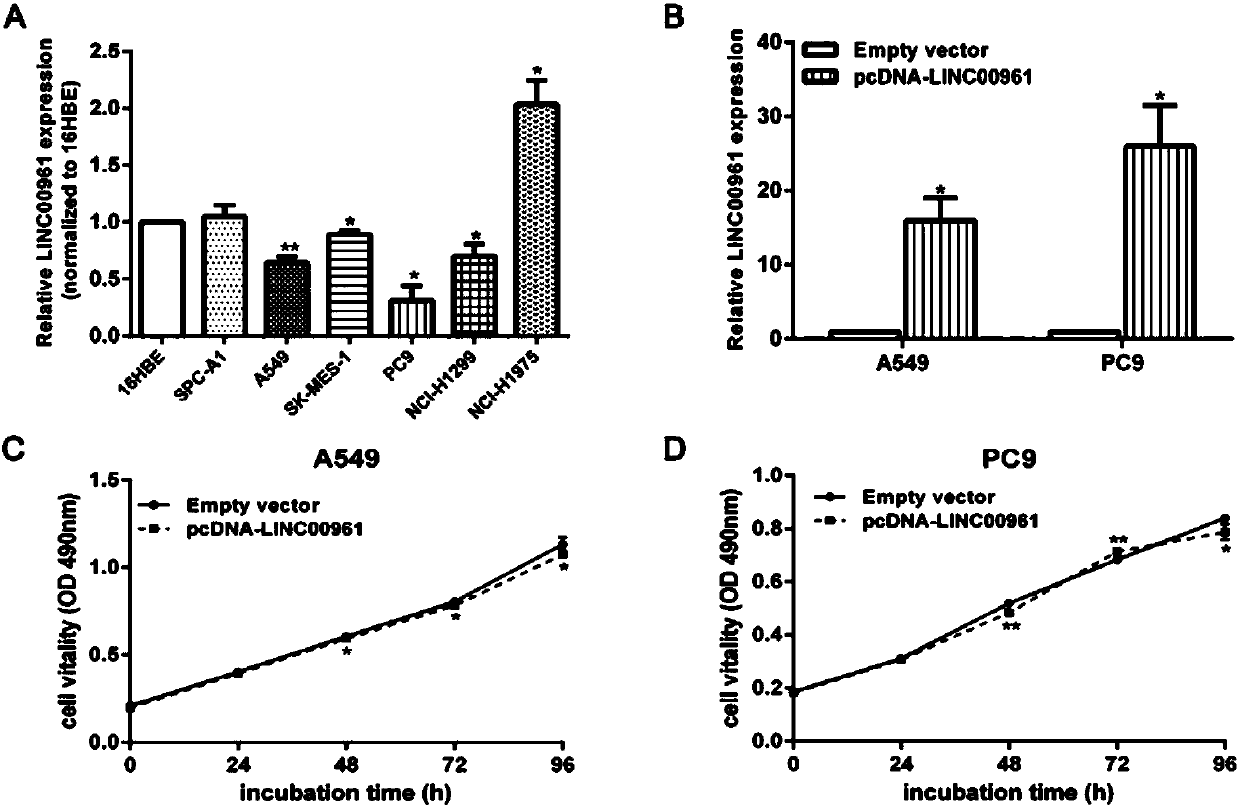
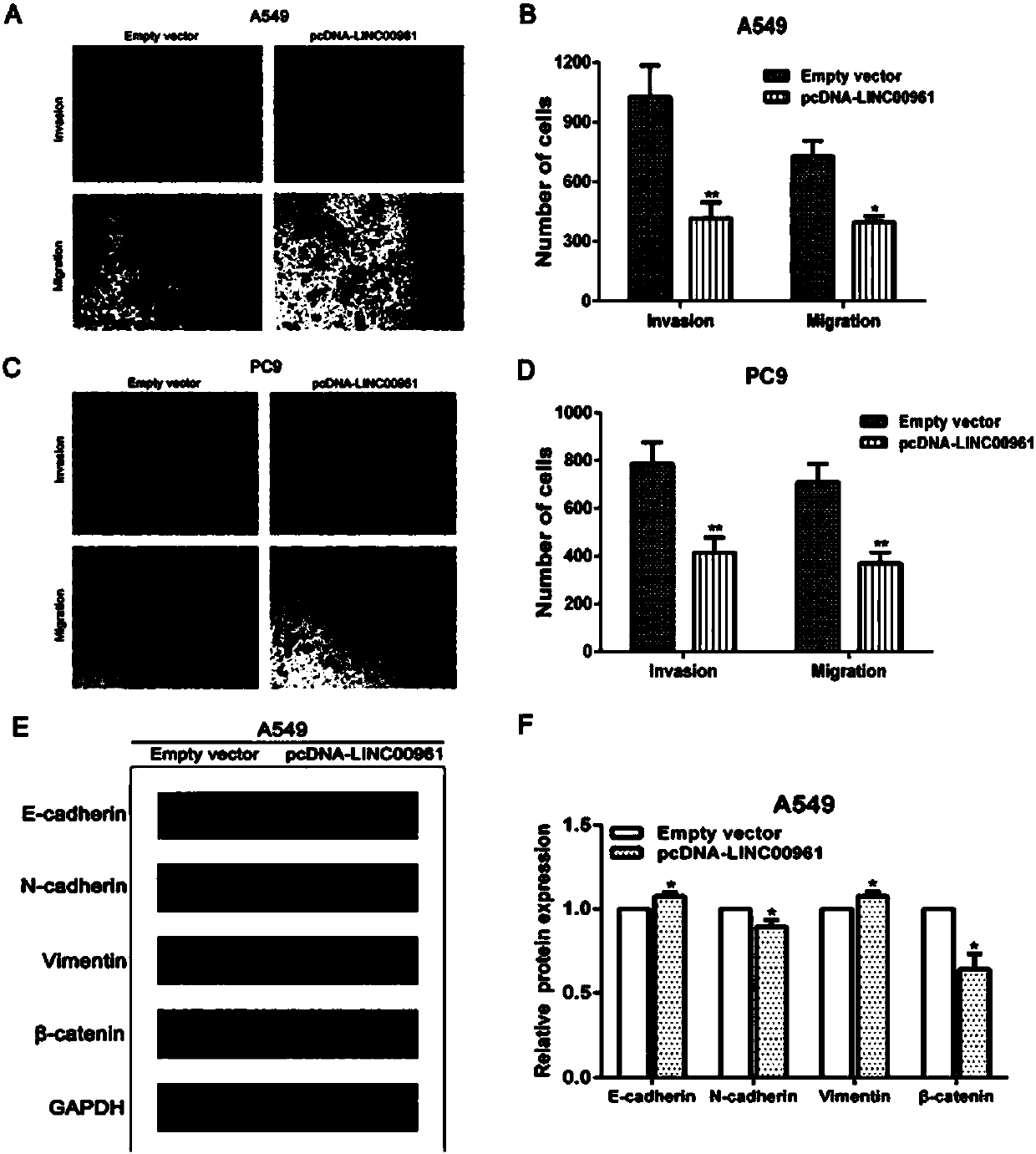
Long non-coding RNA (Ribose Nucleic Acid) and application thereof to diagnosis/treatment of non-small-cell lung cancer
InactiveCN105950628AOrganic active ingredientsMicrobiological testing/measurementSmall cell lung carcinoma cellInvasion and migration
The invention belongs to the field of gene engineering, and particularly relates to application of LINC00961 to prediction of non-small-cell lung cancer (NSCLC) prognosis and treatment of target spot medicine. In the NSCLC, down-regulation of the LINC00961 is closely related to clinical stages and tumor sizes, and is closely related to patient prognosis at the same time. The invasion, migration and the like of NSCLC cells are influenced by changing expression of the LINC00961, so that the LINC00961 expression is enhanced, and the invasion and migration of the NSCLC cells can be restrained.
Owner:JIANGYIN PEOPLES HOSPITAL
Nucleic acid aptamer for classification of different-subtype non-small cell lung cancers and screening method thereof
InactiveCN101914543AMicrobiological testing/measurementIndividual particle analysisScreening methodTumor therapy
The invention discloses a nucleic acid aptamer for classification of different-subtype non-small cell lung cancers and a screening method thereof. The nucleic acid aptamer provided by the invention is a DNA segment which comprises nucleotide shown as any one of sequences 1 to 9 in a sequence table. The nucleic acid aptamer can be applied to the classification of the different-subtype non-small cell lung cancers. By using the nucleic acid aptamer of the invention, the differentiation of the different-subtype non-small cell lung cancers can be realized on a molecular response signal under the condition that tumor markers of the non-small cell lung cancers are unknown. When used for identifying a target combined with the nucleic acid aptamer, the nucleic acid aptamer is favorable for finding out the tumor markers of the different-subtype non-small cell lung cancers, performing earlier diagnosis and more accurate classification of the non-small cell lung cancers and finding out new medicament acting target points for tumor therapy.
Owner:INST OF CHEM CHINESE ACAD OF SCI
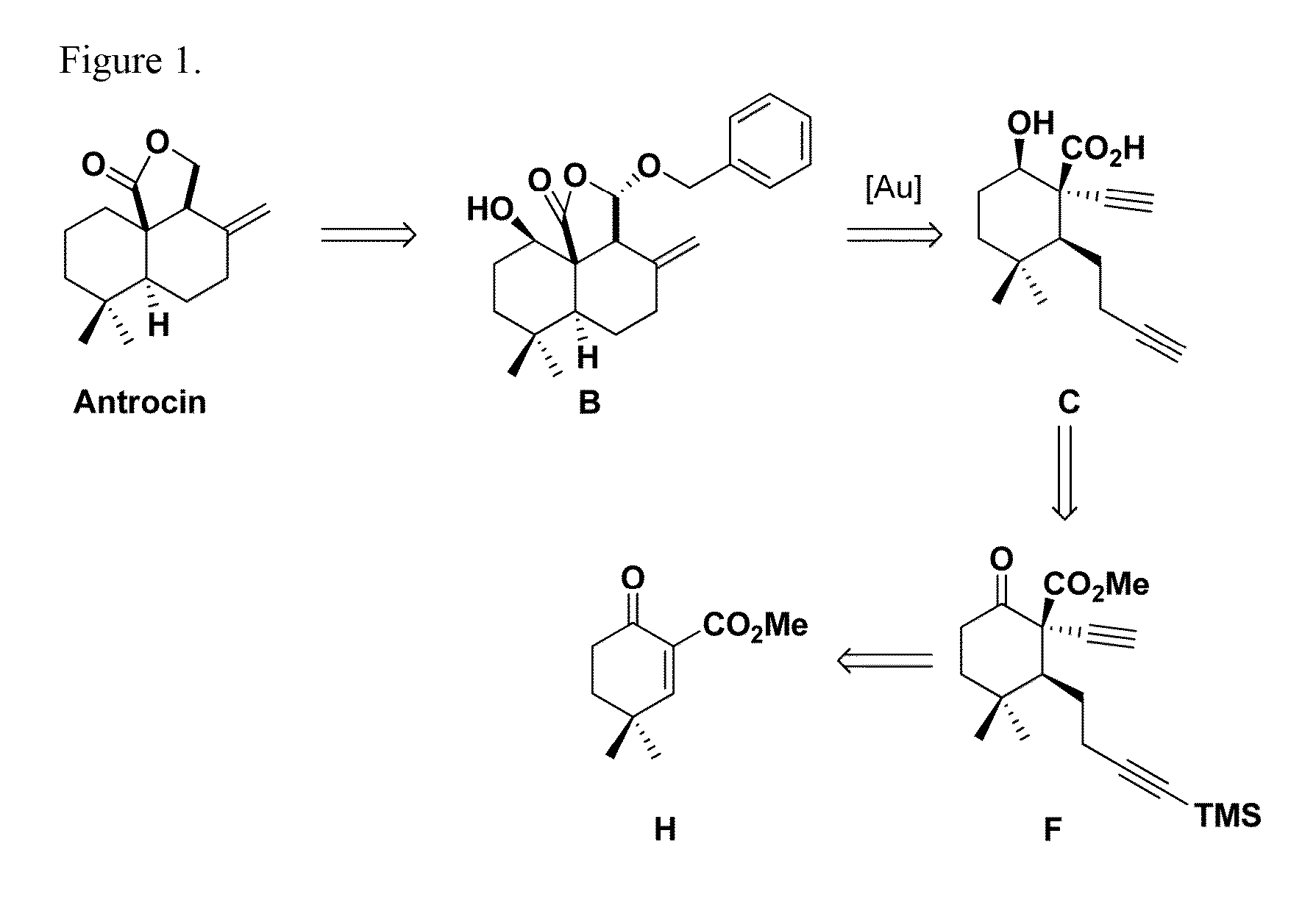
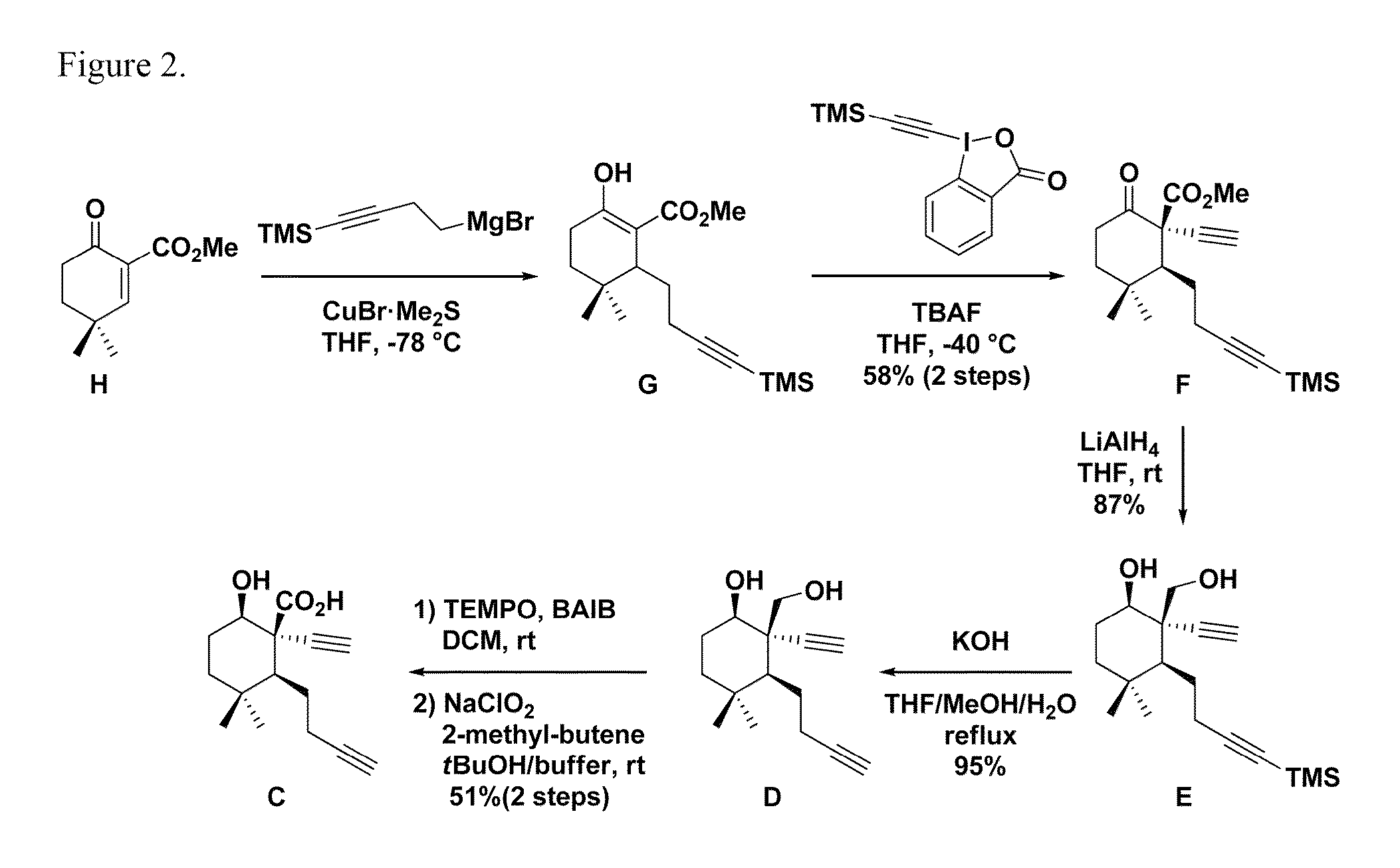
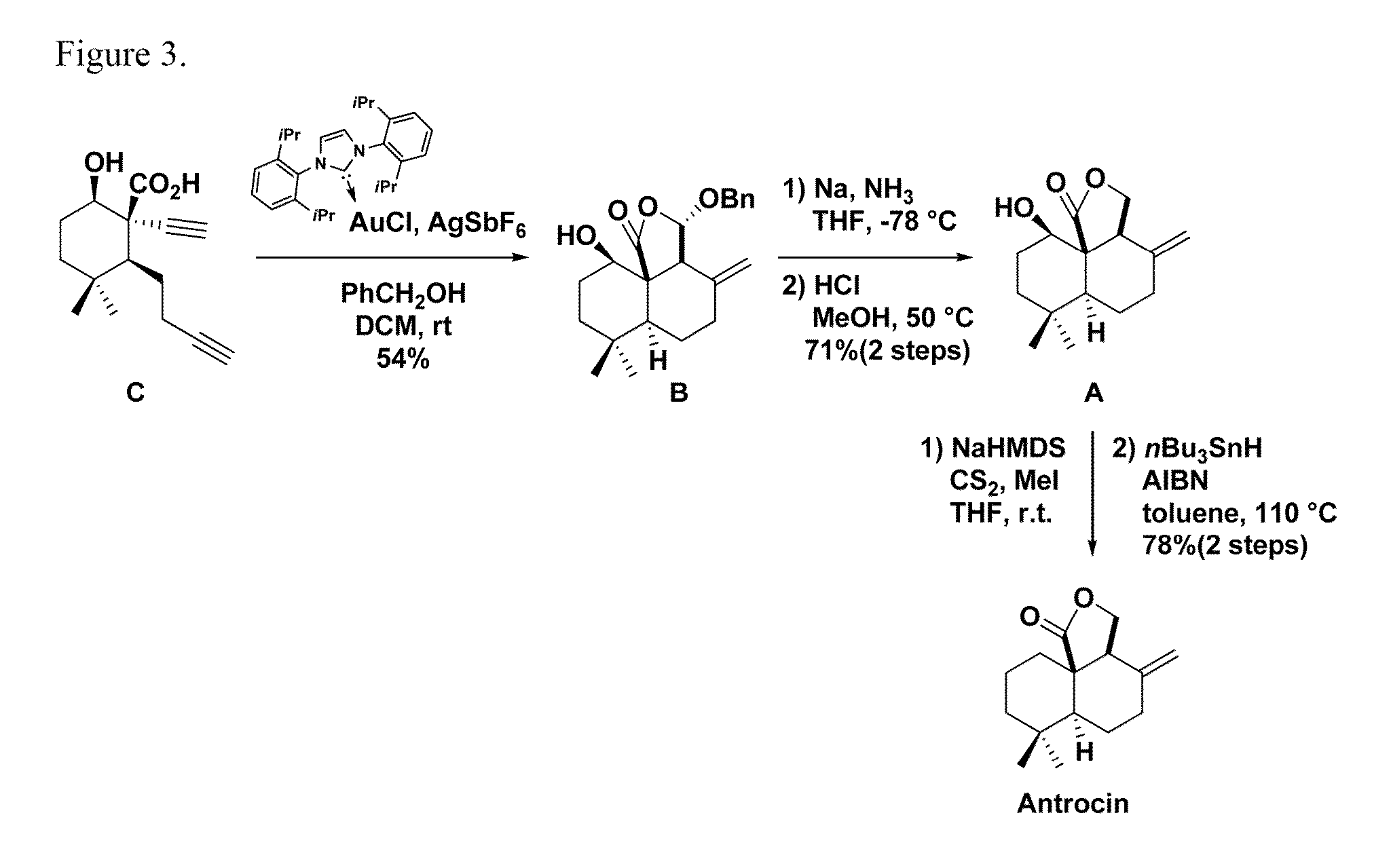
Method for chemical synthesis of antrocin and use thereof for suppressing non-small cell lung cancer
InactiveUS20140350096A1Growth inhibitionInhibit cell proliferationBiocideOrganic chemistryChemical synthesisMedicine
The present invention provides a method for preparing antrocin of pharmaceutically acceptable salts thereof via a series of gold-catalyzed cyclization to construct the (6-6-5) tricyclic core frame. The present invention also provides a use of a composition in preparing drugs for suppressing growth of non-small cell lung cancer cells, wherein the composition comprises an effective amount of antrocin or pharmaceutically acceptable salts thereof and a pharmaceutically acceptable carrier.
Owner:YANG ZHEN +3
Small molecule peptide and use thereof
InactiveCN101486754AImprove diagnosis rateStrong specificityTissue cultureCarrier-bound/immobilised peptidesSmall cell lung carcinoma cellOrganic chemistry
The invention provides a small molecule peptide with the amino acid sequence of cdGIGPQc (SEQ NO.1); and the invention can be prepared by a solid-phase synthesis method. The small molecule peptide has specific adhesion action to non-small cell lung cancer cells and can be used for separating the non-small cell lung cancer cells. The invention also provides a resin bead which is formed by a resin bead with an NH2 active group on the surface and the small molecule peptide by -CO-NH- connection. The resin bead has very high specificity for the non-small cell lung cancer cells, can be used for assisting chest hydrocele desquamate cell test method in diagnosing non-small cell lung cancer, and improves the diagnosis rate to be 13.51%.
Owner:SOUTHERN MEDICAL UNIVERSITY
Use of rotenone as non-small cell lung cancer cell sensitizer
InactiveCN101904837ANo obvious side effectsOrganic active ingredientsAntineoplastic agentsSide effectNormal cell
The invention relates to novel use of a mitochondrial respiratory chain inhibitor rotenone in reversing the tolerance of non-small cell lung cancer cells to targeted antineoplastic agents. The rotenone serves as a non-small cell lung cancer cell sensitizer and can reverse the tolerance of the non-small cell lung cancer cells to the targeted antineoplastic agents. The invention shows the combination of rotenone compounds and TRAIL has no obvious toxic or side effect on normal cells and is a novel medicinal composition used for treating the non-small cell lung cancer.
Owner:NANJING UNIV
Novel application of PAK3 as biological marker of neuroendocrine tumor
InactiveCN101520457AMature technology platformProcess specificationMicrobiological testing/measurementColor/spectral properties measurementsPheochromocytomaAbnormal tissue growth
The method relates to novel application of PAK3 as a biological marker of a neuroendocrine tumor. Through protein expression level detection of PAK3 on thymic carcinoid, pheochromocytoma, parathyroid adenoma, pituitary tumor, adrenal adenoma, cell strains of islet cell tumor, cell strains of small cell lung cancer and other neuroendocrine tumor tissues and cells, the PAK3 highly expresses and covers neuroendocrine tumors common in clinics and relates to each tissue and gland of body; the novel application initially finds that the PAK3 is relevant to tumors, in particular to the neuroendocrine tumor; the novel application discloses that the PAK3 can obviously increase the transferring capacity of the cell and shows that the PAK3 plays an important role in the developing process of the neuroendocrine tumor; and the result shows that the PAK3 can be used as the biological marker of the neuroendocrine tumor.
Owner:宁光
Preparation method of Car-NK cell for small cell lung cancer
InactiveCN107557335ASufficient and convenient sourceReduce recurrenceMammal material medical ingredientsFermentationEvery Three DaysFicoll
The invention discloses a preparation method of a Car-NK cell for small cell lung cancer. The method comprises the following steps: collecting peripheral blood of a patient and isolating an NK cell with an Ficoll method; placing a peripheral blood mononuclear cell in an NK medium, adjusting the cell concentration, placing the cell in an incubator for continuous culture for 48-72 h, and collectingan adherent cell; adding the cell to the NK medium for continuous culture for 12-15 days, and supplementing a culture solution once every three days; transfecting the NK cell with lentivirus and performing multiplication culture to obtain the Car-NK cell; preparing a small cell lung cancer cell preparation.
Owner:ANHUI HUIEN BIOTECH
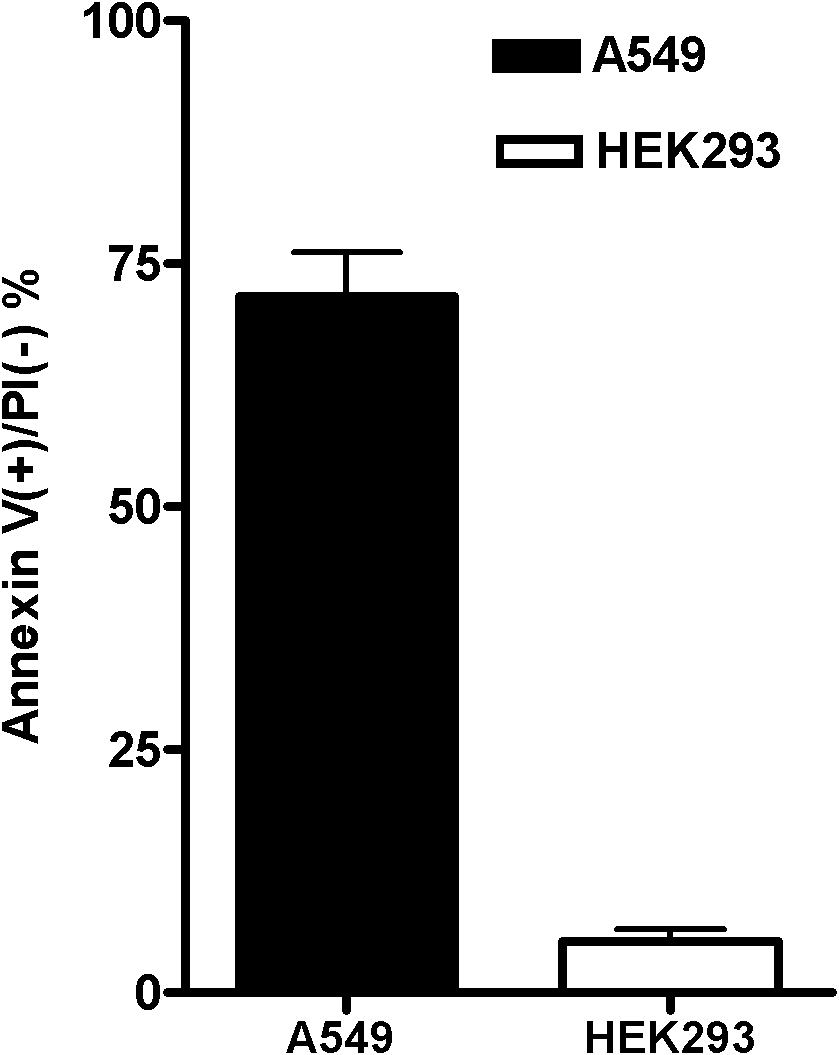
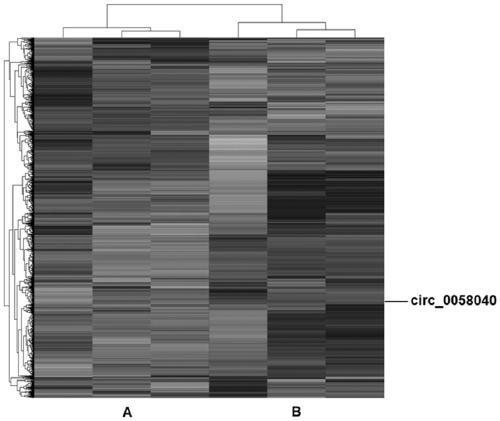
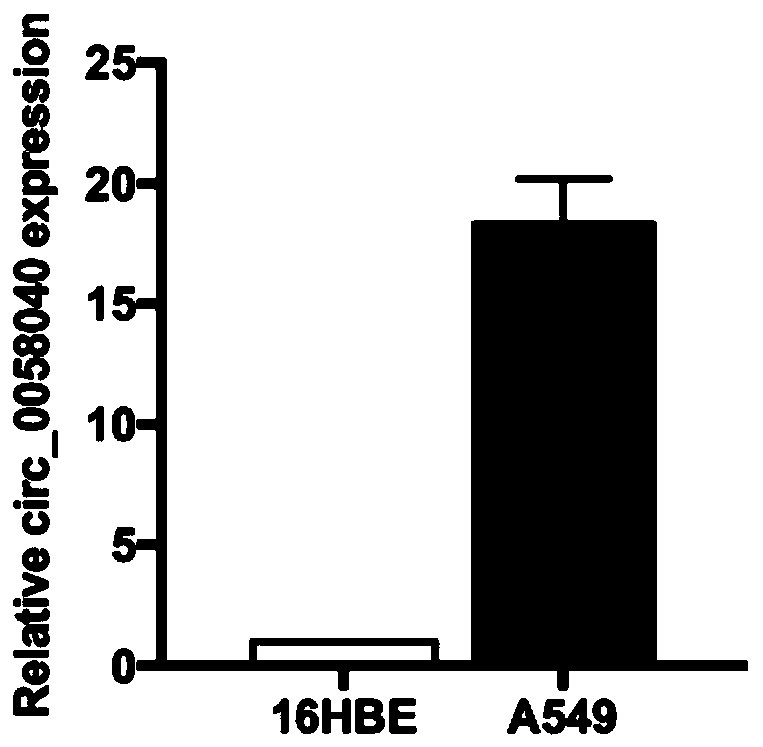
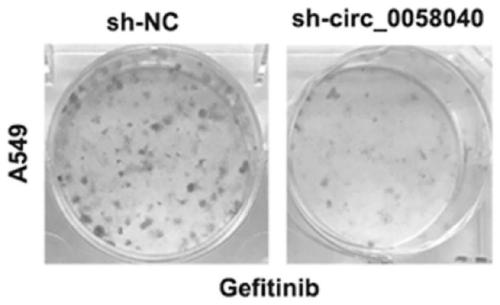
Circular RNA and application thereof
ActiveCN111378664AIncreased sensitivityHigh transfection efficiencyOrganic active ingredientsRespiratory disorderApoptosisOncology
The invention belongs to the field of genetic engineering and particularly relates to an application of a circular RNA circ-0058040 to preparation of a drug for treating a non-small cell lung cancer.Proliferation, apoptosis and drug susceptibility of a non-small cell lung cancer cell are influenced by changing expression of the circular RNA circ-0058040; the application illustrates that the proliferation of the non-small cell lung cancer cell can be inhibited by the expression of the circular RNA circ-0058040; and the susceptibility of the non-small cell lung cancer cell to a targeted drug can be enhanced.
Owner:THE SECOND AFFILIATED HOSPITAL OF NANJING MEDICAL UNIV
Compounds and methods of prophylaxis and treatment regarding nicotinic receptor antagonists
ActiveUS9108949B2Good effectIncrease awarenessHeavy metal active ingredientsNervous disorderMedicineNK1 receptor antagonist
Owner:INTRA CELLULAR THERAPIES INC
Application of anti-human immunodeficiency virus (HIV) drug amprenavir in preparation of antitumor drug
InactiveCN103536605AShorten the development cycleReduce R&D investmentOrganic active ingredientsAntineoplastic agentsL929 cellFibroblastic Tumor
The invention discloses application of anti-human immunodeficiency virus (HIV) drug amprenavir in preparation of an antitumor drug. The anti-HIV drug amprenavir identified by food and drug administration (FDA) is taken as a material; the killing effect of the amprenavir on a tumor cell is detected by adopting a microwave theory and techniques (MTT) method. An experiment proves that the amprenavir has a significant inhibiting effect on multiplication of a plurality of tumor cells, such as a breast cancer michigan cancer foundation (MCF-7) cell, a non-small cell lung cancer A549 cell, a mouse embryonic fibroblast glioblastoma multiforme L929 cell, a colon cancer HT29 cell, a brain glioma U87 cell and the like, so as to clarify new use of an old drug. Therefore, foundation is provided for development of a novel anti-tumor drug.
Owner:SICHUAN UNIV
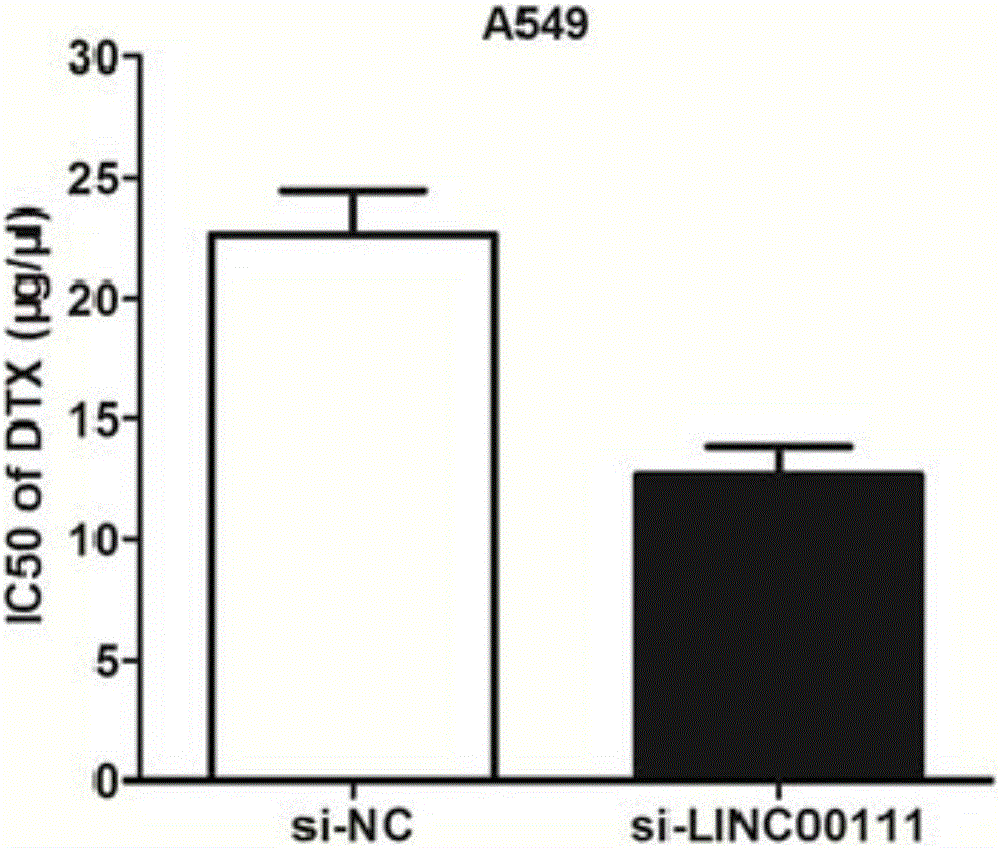
Long non-coding RNA and applications of long non-coding RNA in diagnosis/treatment of non-small cell lung cancer
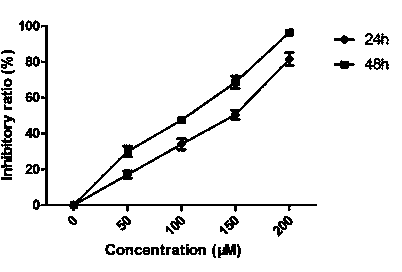
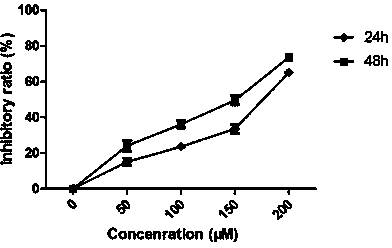
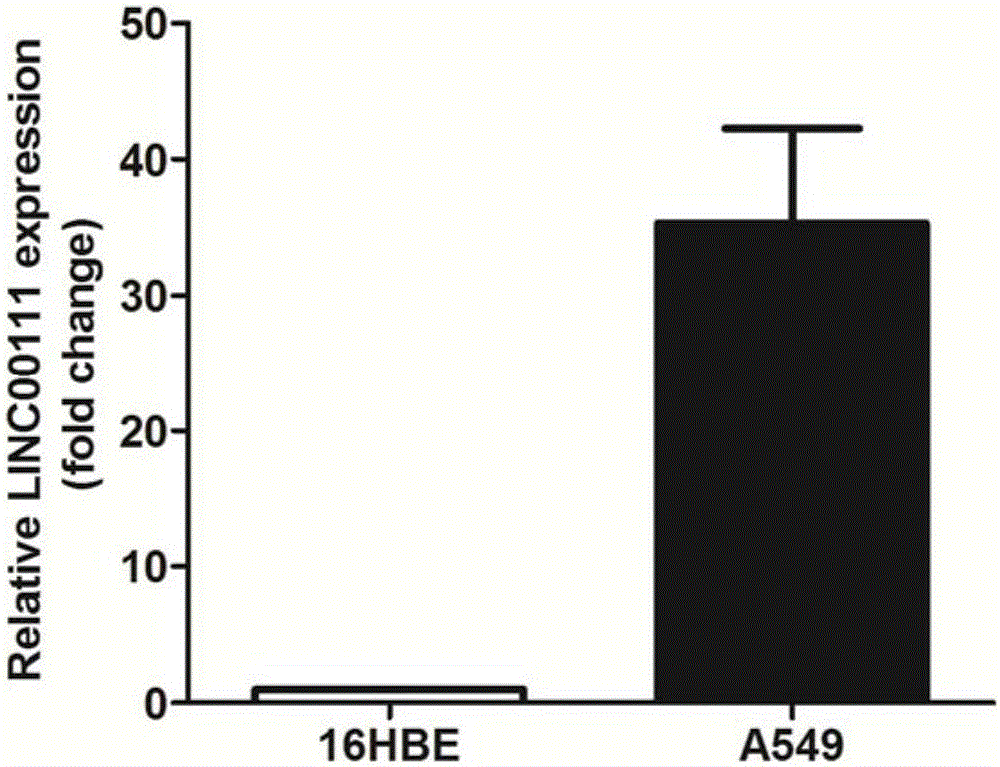
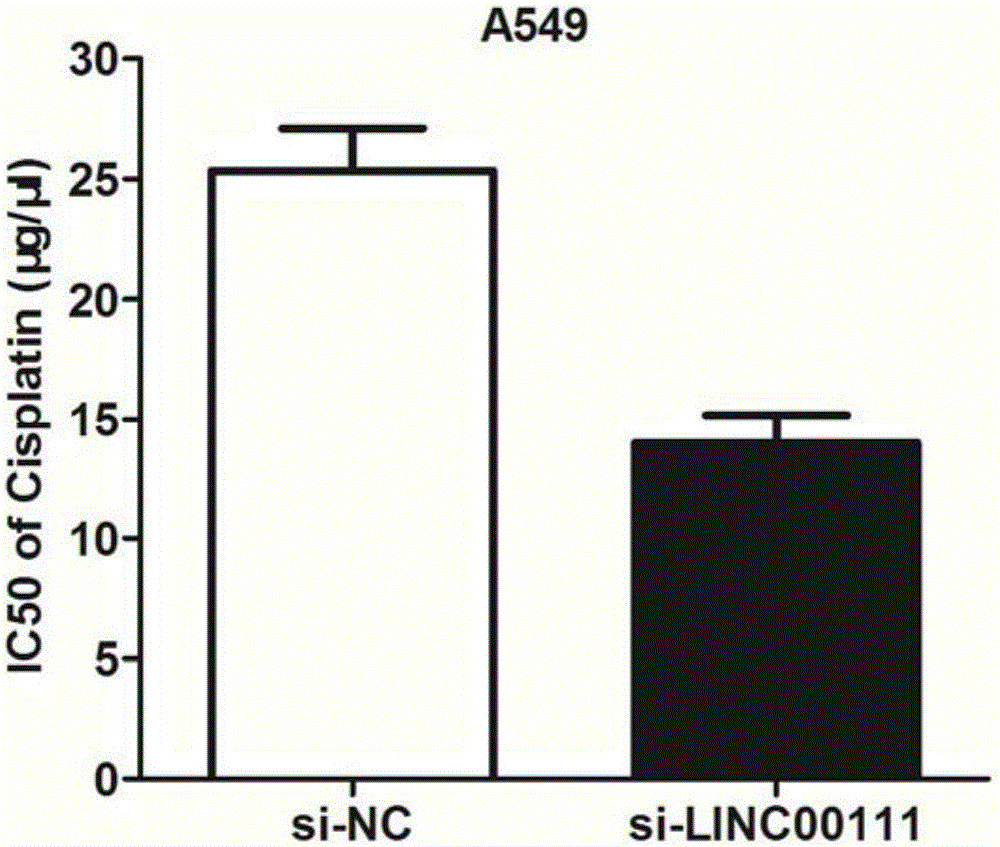
ActiveCN106244592AOrganic active ingredientsMicrobiological testing/measurementApoptosisSmall cell lung carcinoma cell
The invention belongs to the field of genetic engineering, and particularly relates to applications of LINC00111 in preparation of drugs for predicting the prognosis and the target point of non-small cell lung cancer, and treatment. According to the present invention, the expression of LINC00111 in NSCLC is up-regulated, such that the influence on the invasion, the migration and the like of non-small cell lung cancer cells can be produced by changing the expression of LINC00111, and the non-small cell lung cancer cell proliferation can be inhibited and the apoptosis can be induced by knocking down the expression of LINC00111.
Owner:克孜勒苏柯尔克孜自治州人民医院
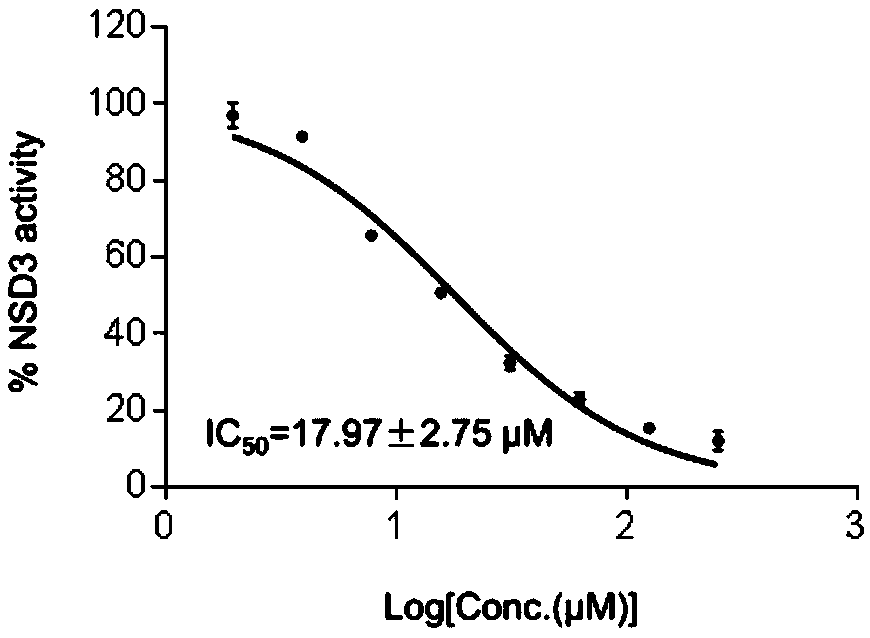
Compound C6 as histone methyltransferase NSD3 activity inhibitor and application thereof
ActiveCN109223794AEnhanced inhibitory effectAntiproliferativeAntineoplastic agentsHeterocyclic compound active ingredientsChemical structurePurine
A C6 compound as histone methyltransferase NSD3 activity inhibitor and its pharmaceutical use are disclosed. C6 has a chemical structure represented by formula I. Chemical name is 6-Amino-9-(2-(naphthalen-1-Yloxy) ethyl)-9H-Purine-8-Thiol; At least one of that compound C6 or its hydrate, pharmaceutically acceptable salt, tautomers, stereoisomer. And precursor compounds is used as an active ingredient for preparing an antineoplastic drug. The pharmaceutical use of the compound C6 or its hydrate, the pharmaceutically acceptable salt, the tautomers, the stereoisomers. And the precursor compoundsare described. Experiments show that the compound C6 of the invention can effectively inhibit the NSD3 enzyme activity. And the IC50 value of the enzyme level is 17.97 +-2. 75[mu]mol / L. And significantly inhibited the proliferation of non-small cell lung cancer cell lines H460, H1299 and H1650. The compound of the invention has the effect of inhibiting tumor cell proliferation. And is expected tobe used as an active ingredient for preparing an anti-tumor drug. And has pharmaceutical prospect.
Owner:普美瑞(常州)生物科技有限公司
1,2,3,4,6-O-pentagalloylglucose germanium complex and preparation method and application thereof
ActiveCN108864236ANovel structureThe synthesis process is simpleSugar derivativesSugar derivatives preparationPancreatic cancer cellHuman breast
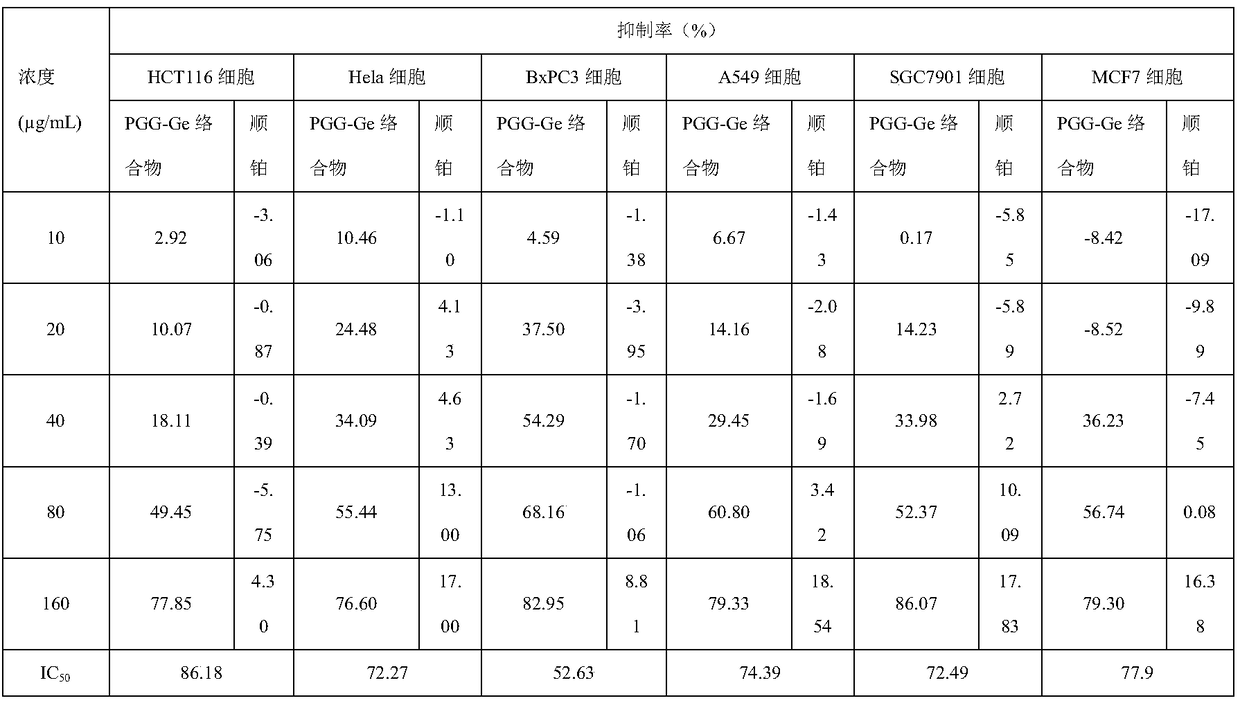
The invention discloses a 1,2,3,4,6-O-pentagalloylglucose germanium complex and a preparation method and application thereof. The 1,2,3,4,6-O-pentagalloylglucose germanium complex is formed by complexing of 1,2,3,4,6-O-pentagalloylglucose and germanium ions. The invention further discloses a specific synthesis process and application of the 1,2,3,4,6-O-pentagalloylglucose germanium complex in preparation of lung cancer treatment or prevention medicine. The synthesized compound is novel in structure, and the synthesis process is simple. The compound has good inhibiting effect on human colon cancer cells HCT116, human cervical cancer cells Hela, human pancreatic cancer cells BxPC3, human small cell lung cancer cells A549, human gastric cancer cells SGC7901 and human breast cancer cells MCF7.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
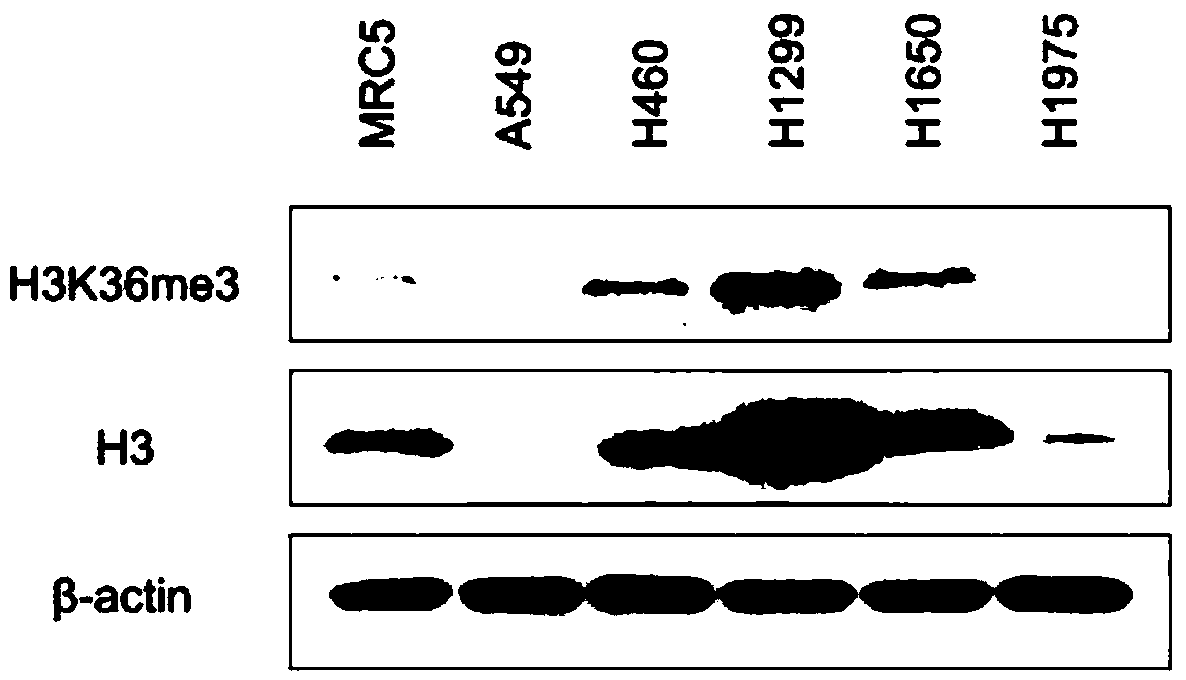
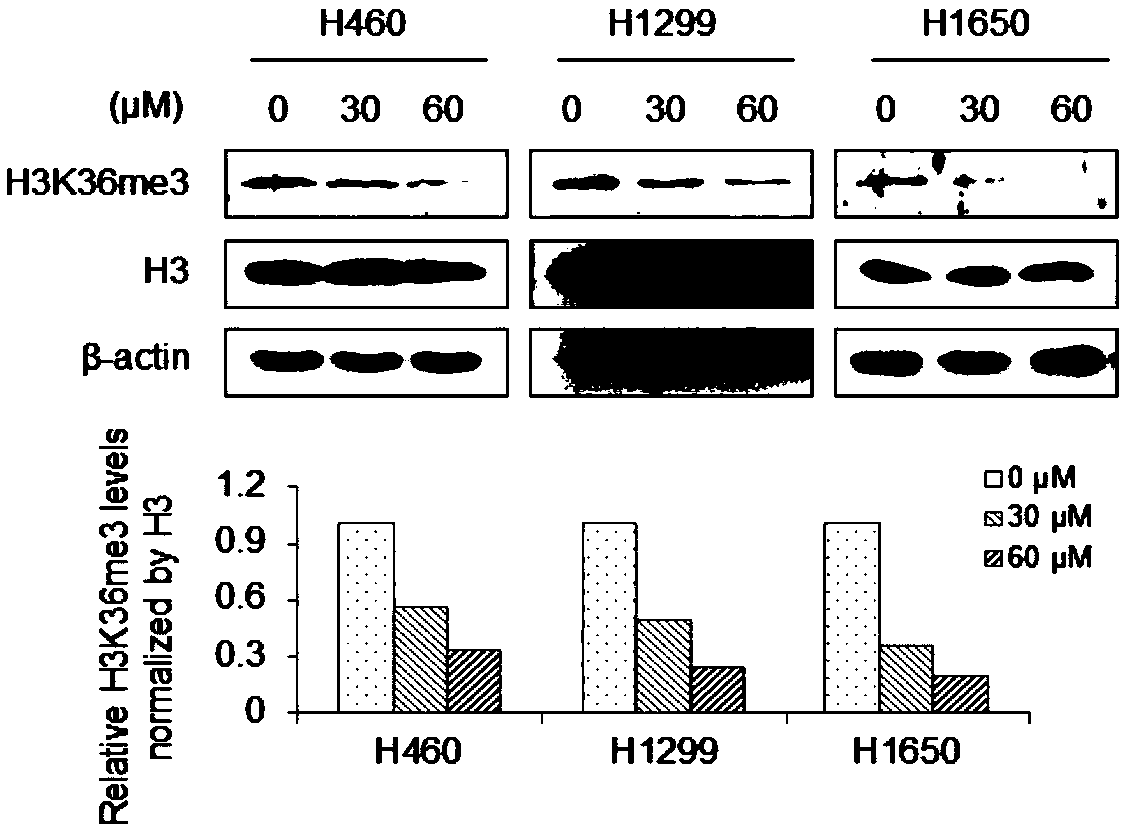
Compound A8 used as histone transmethylase NSD3 activity inhibitor and application thereof
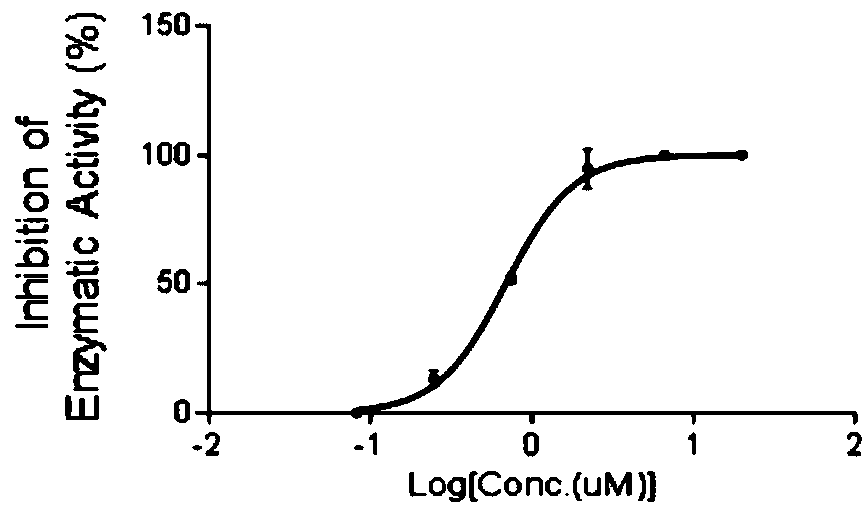
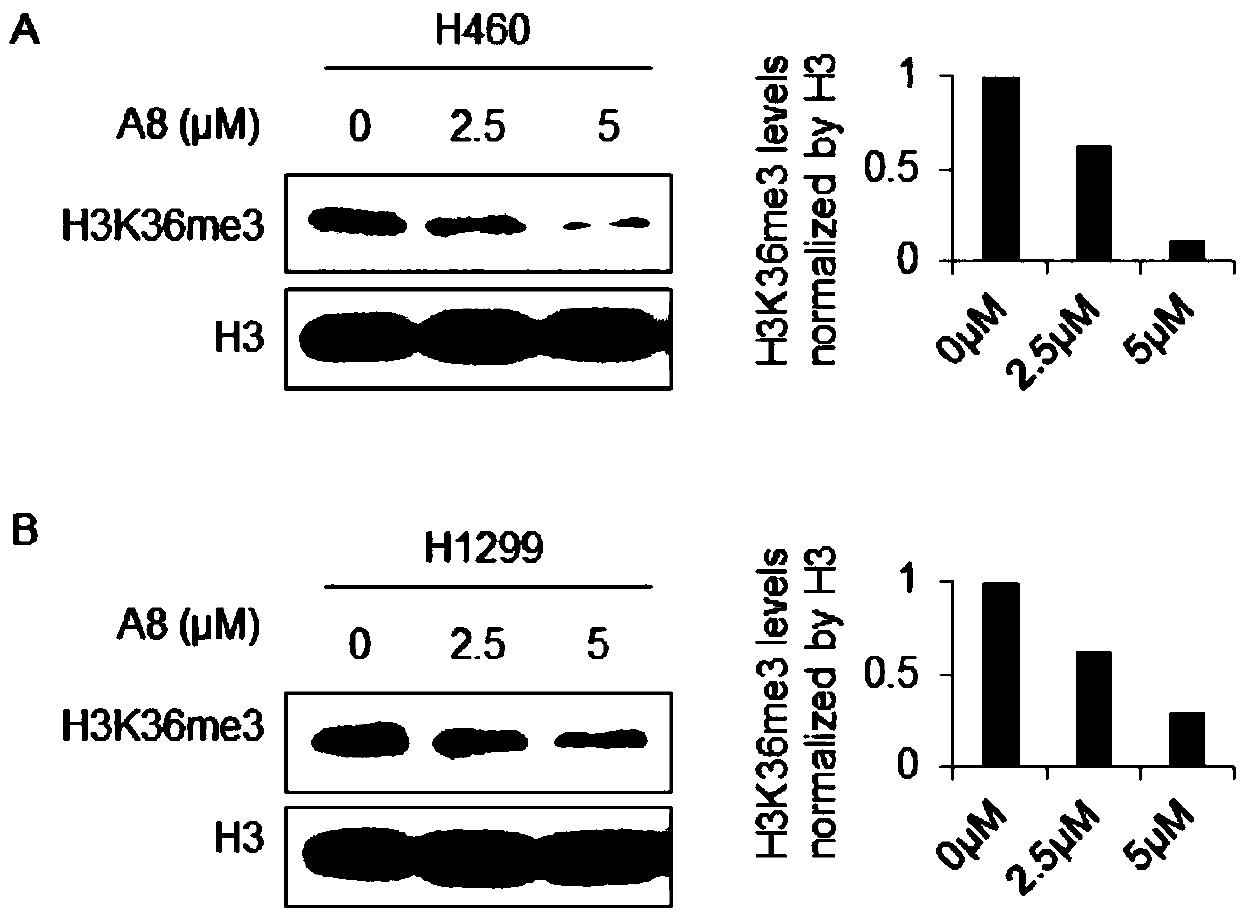
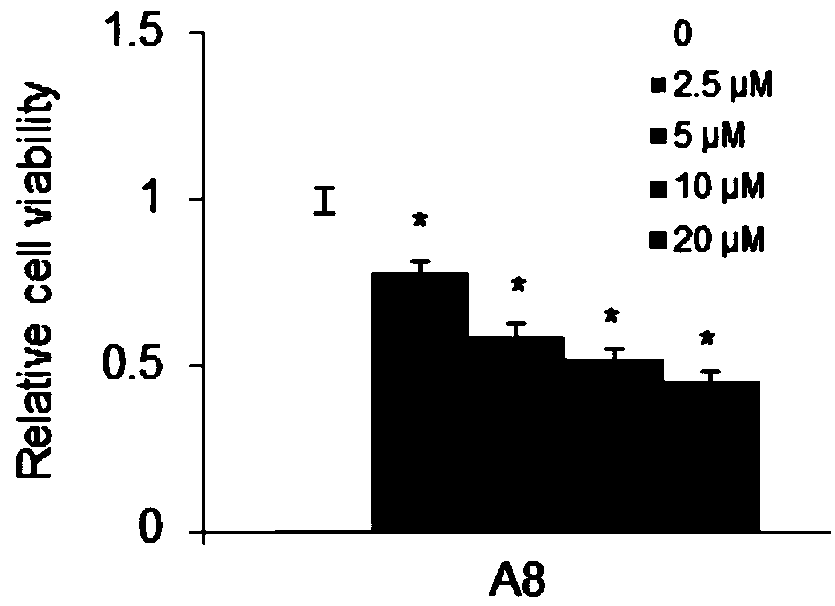
ActiveCN108853110AValidation of antitumor activityEnhanced inhibitory effectAntineoplastic agentsHeterocyclic compound active ingredientsChemical structurePurine
The invention discloses a compound A8 used as a histone transmethylase NSD3 activity inhibitor and a medicine purpose thereof. The compound A8 has a chemical structure shown as a formula I in description, and the chemical name is 6-amino-9-(2-(p-tolyloxy)ethyl)-9H-purine-8-thiol. The medicine purpose refers to the preparation of anti-tumor medicine by using at least one of the compound A8 or hydrates, pharmaceutically acceptable salt, dynamic isomers, stereoisomers and precursor compounds as active ingredients. Experiments show that the compound A8 can effectively inhibit the NSD3 enzyme activity; the enzyme level IC50 value is 0.69+ / -0.06mumol / L; the growth and proliferation of a non-small-cell lung cancer cell line H460 can be obviously inhibited. The compound has the effect of inhibiting the tumor cell proliferation, is hopeful to being used as an active ingredient for preparing the anti-tumor medicine and has medicine prospects.
Owner:普美瑞(常州)生物科技有限公司
Method for chemical synthesis of antrocin and use thereof for suppressing non-small cell lung cancer
InactiveUS9045450B2Inhibit cell proliferationOrganic active ingredientsOrganic chemistryChemical synthesisMedicine
The present invention provides a method for preparing antrocin of pharmaceutically acceptable salts thereof via a series of gold-catalyzed cyclization to construct the (6-6-5) tricyclic core frame. The present invention also provides a use of a composition in preparing drugs for suppressing growth of non-small cell lung cancer cells, wherein the composition comprises an effective amount of antrocin or pharmaceutically acceptable salts thereof and a pharmaceutically acceptable carrier.
Owner:YANG ZHEN +3
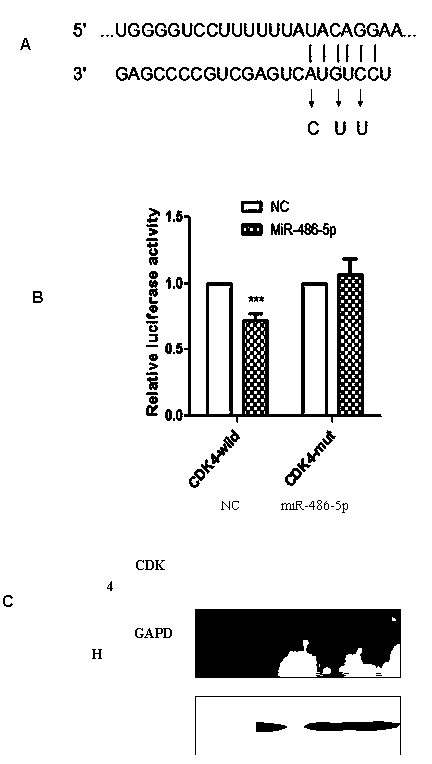
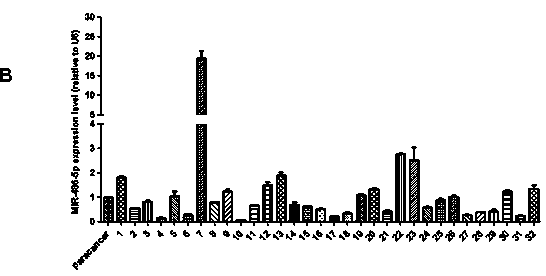
Application of miR-486-5p in non-small cell lung carcinoma cell line
InactiveCN104069506AInhibition of translationAvoid degradationGenetic material ingredientsAntineoplastic agentsH1299 cellDrug target
The invention relates to application of miR-486-5p in a non-small cell lung carcinoma cell line. The miR-486-5p performs specific low-expression in a non-small cell lung carcinoma cell and a series of lung carcinoma tissue clinical specimen; in an H1299 cell, the over-expressed miR-486a-5p can inhibit cell proliferation of H1299 and inhibit G1 / S conversion to block the cell in the G1 phase. Experiments based on bioinformatics analysis discovers that the miR-486a-5p is complementary with 3'-UTR () of a target gene mRNA (messenger ribonucleic acid), so that translation of the mRNA of the target gene can be inhibited or the mRNA of the target gene can be directly degraded; furthermore, western blotting and other experiments prove that the CDK4 gene is the target gene of the miR-486a-5p. The experiment first proves that the CDK4 gene is the garget gene of miR-486a-5p, and the miRNA is utilized to diagnose and treat non-small cell lung carcinoma in clinical application, and a certain application value is created for providing the aspect of drug targets.
Owner:SHANGHAI UNIV
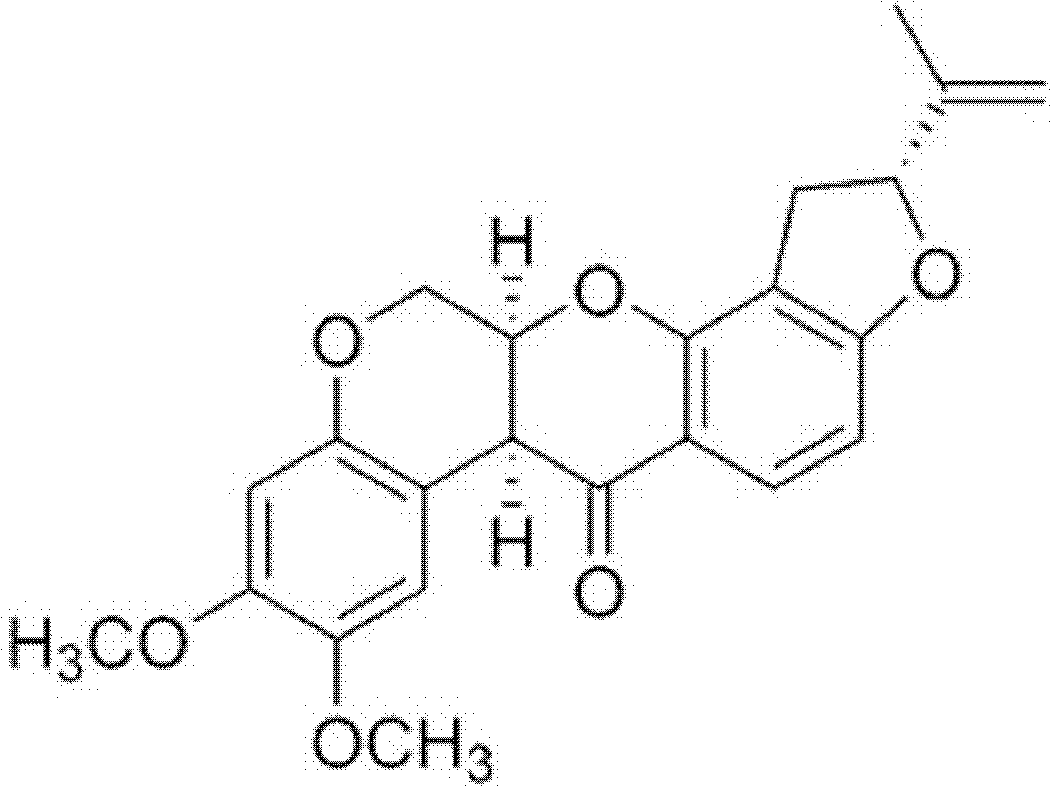
2-phenyl-4H-benzo [1, 3] oxazine derivative containing 3-phenylfuran as well as preparation and application thereof
PendingCN113956246AEasy to operateMild conditionsOrganic active ingredientsOrganic chemistry methodsOncologyHuman breast
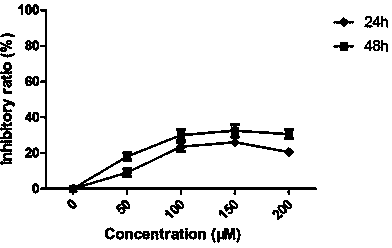
The invention belongs to the technical field of pharmaceutical chemicals, and particularly relates to a 2-phenyl-4H-benzo [1, 3] oxazine derivative containing 3-phenylfuran as well as preparation and application thereof. The 2-phenyl-4H-benzo [1, 3] oxazine derivative containing 3-phenylfuran provided by the invention can be prepared only through one-step reaction. According to the invention, compounds which can be purchased in the market or are easy to synthesize are used as raw materials, and the reaction has the characteristics of simple operation, mild conditions, few steps, fast reaction speed, low cost, few generated wastes, high atom economy and the like. Meanwhile, the derivative shows a certain inhibitory activity on four tumor cells (human osteosarcoma cells, human colon cancer cells, human non-small cell lung cancer cells and human breast cancer cells), and is expected to be prepared into drugs for inhibiting tumor cells, especially drugs for inhibiting non-small cell lung cancer cells.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Aziridinyl quinone antitumor agents based on indoles and cyclopent[b]indoles Aziridinyl quinone antitumor agents based on indoles and cyclopent[b]indoles](https://images-eureka.patsnap.com/patent_img/d3d26f69-9bf8-4af9-97bf-bf399977eec9/US20030139609A1-20030724-C00001.png)
![Aziridinyl quinone antitumor agents based on indoles and cyclopent[b]indoles Aziridinyl quinone antitumor agents based on indoles and cyclopent[b]indoles](https://images-eureka.patsnap.com/patent_img/d3d26f69-9bf8-4af9-97bf-bf399977eec9/US20030139609A1-20030724-C00002.png)
![Aziridinyl quinone antitumor agents based on indoles and cyclopent[b]indoles Aziridinyl quinone antitumor agents based on indoles and cyclopent[b]indoles](https://images-eureka.patsnap.com/patent_img/d3d26f69-9bf8-4af9-97bf-bf399977eec9/US20030139609A1-20030724-C00003.png)

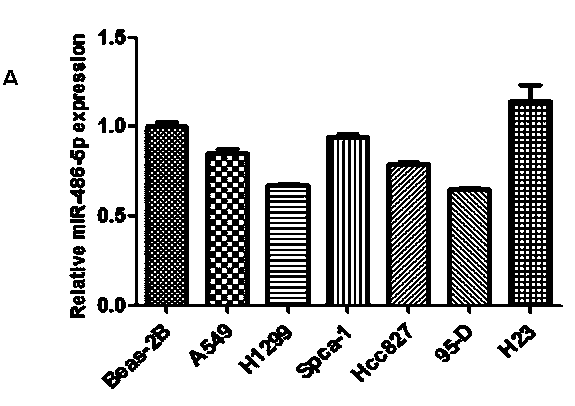
![2-phenyl-4H-benzo [1, 3] oxazine derivative containing 3-phenylfuran as well as preparation and application thereof 2-phenyl-4H-benzo [1, 3] oxazine derivative containing 3-phenylfuran as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/24627b6a-1df8-4a72-a60d-b07d180b35fb/FDA0003318363610000011.png)
![2-phenyl-4H-benzo [1, 3] oxazine derivative containing 3-phenylfuran as well as preparation and application thereof 2-phenyl-4H-benzo [1, 3] oxazine derivative containing 3-phenylfuran as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/24627b6a-1df8-4a72-a60d-b07d180b35fb/FDA0003318363610000021.png)
![2-phenyl-4H-benzo [1, 3] oxazine derivative containing 3-phenylfuran as well as preparation and application thereof 2-phenyl-4H-benzo [1, 3] oxazine derivative containing 3-phenylfuran as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/24627b6a-1df8-4a72-a60d-b07d180b35fb/BDA0003318363620000021.png)