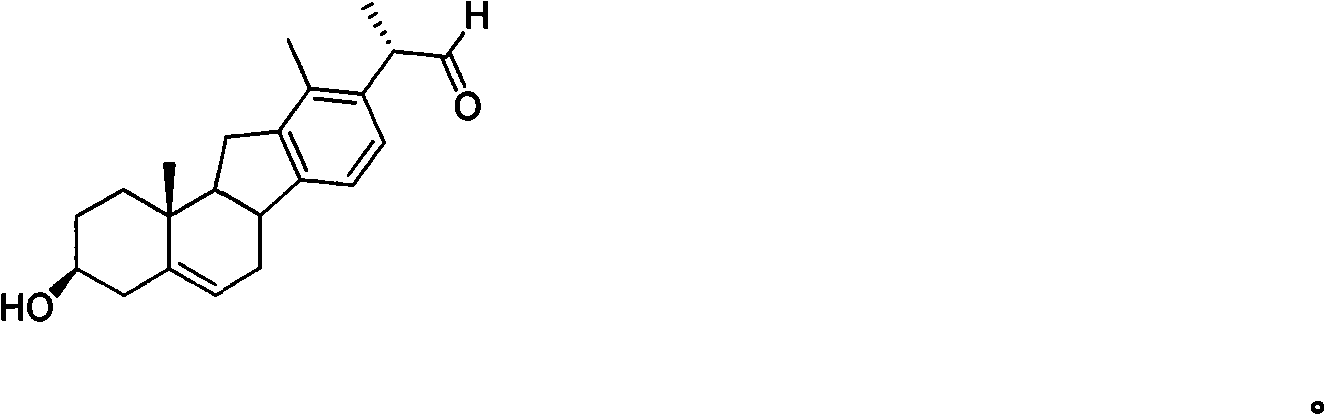
Veratramine degradation product veratrum fluorene aldehyde and the derivatives thereof, as well as the preparation and application thereof
A degradation product, veratramine technology, applied in the direction of drug combinations, steroids, medical preparations containing active ingredients, etc., can solve the problem of high toxicity of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dissolve 2.0g (4.9mmol) of veratramine A in 40mL of toluene, add 5mL of cyclohexanone (48.2mmol) and 2.0g (9.8mmol) of aluminum isopropoxide, raise the temperature to 110°C for 3h, distill off the toluene to obtain Yellow oil, purified by silica gel column to obtain white powder compound 3-carbonyl-Δ 4 - Veratramine B (1.2g), yield 60.3%, melting point 187-189°C.
[0028] 3-Carbonyl-Δ 4 -Structure Confirmation of Veratramine (B):
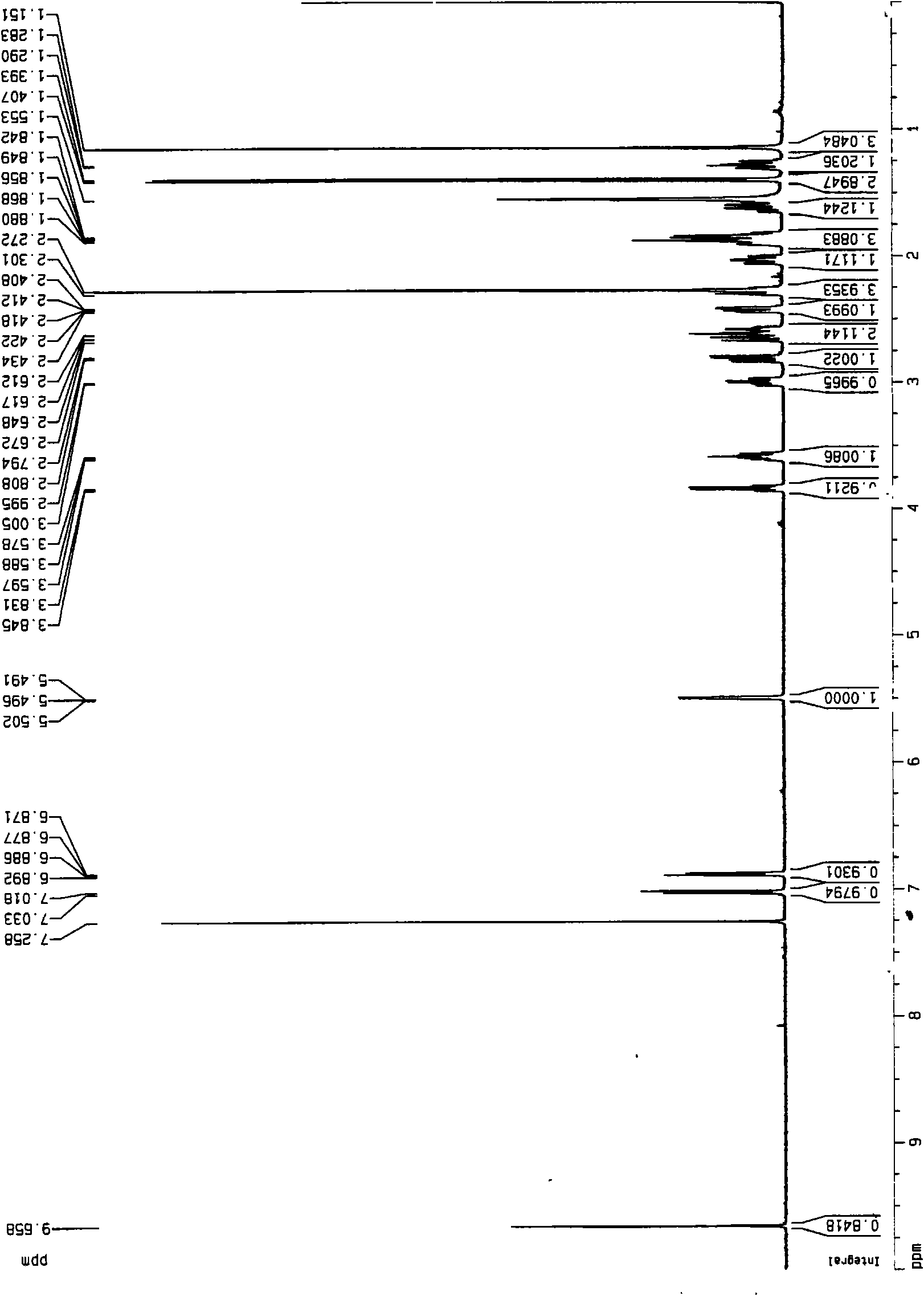
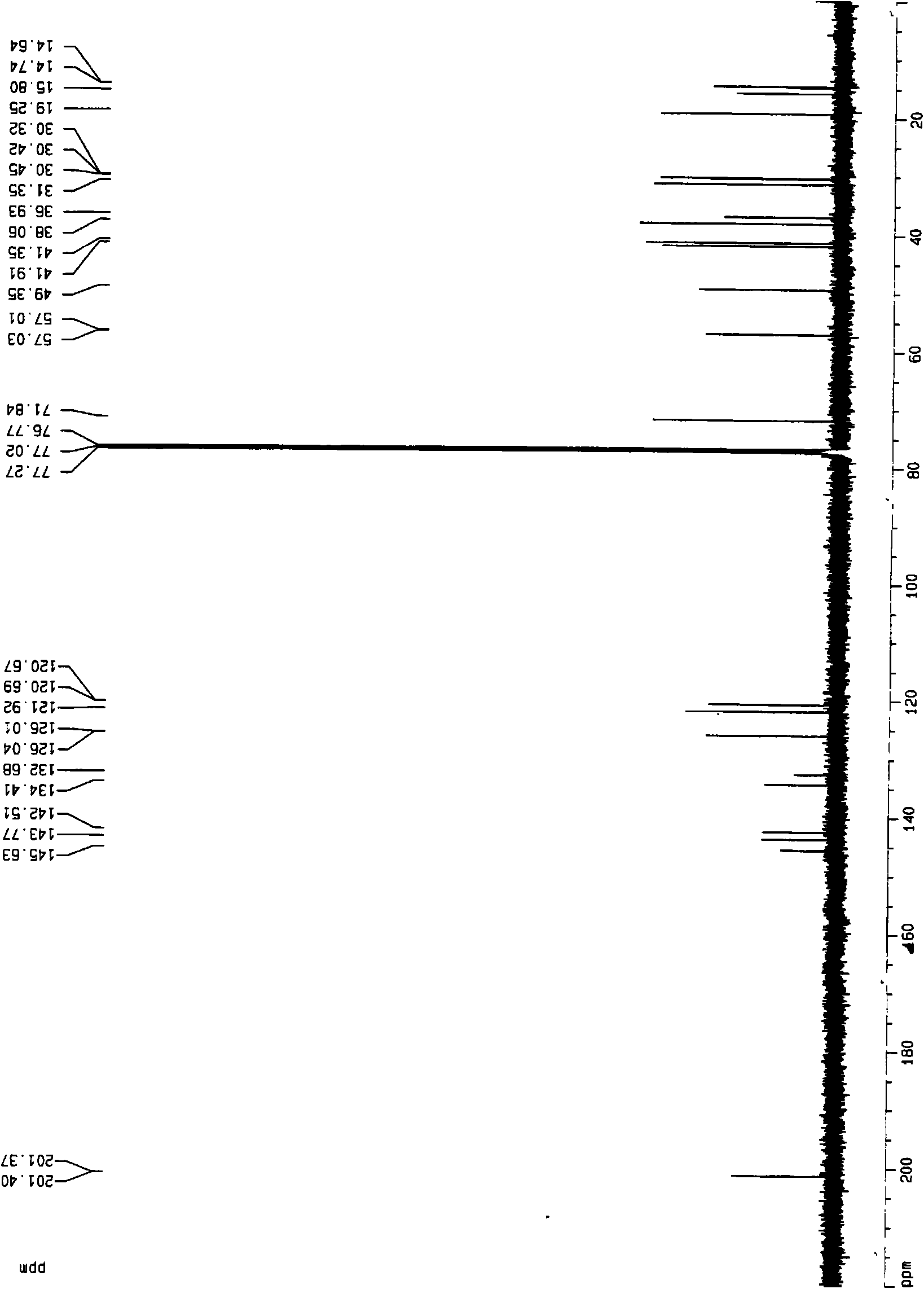
[0029] ESI-MS: m / z 408[M+H] + , 1 H-NMR (600MHz, CDCl 3 , J / Hz) δ: 7.24 (d, 1H, J=7.8, H-16), 6.98 (d, 1H, J=7.8, H-15), 5.83 (s, 1H, H-4), 3.50 ( dd, 1H, J=6.6, 4.8, H-20), 3.26(m, 1H, H-23), 3.06(m, 1H, H-8), 2.92(dd, 1H, J=12, 1.8, H -26 b ), 2.76(q, 1H, H-11 b ), 2.61(m, 1H, H-6 b ), 2.56(m, 1H, H-11 a ), 2.55(m, 1H, H-2 b ), 2.49(d, 1H, J=4.2, H-22), 2.47(m, 1H, H-6 a ), 2.43 (brs, 1H, H-7 b ), 2.40 (brs, 1H, H-2 a ), 2.32 (brs, 3H, H-18), 2.11 (t, 1H, J=11.4, H-26 a ), 2.04(m, 1H, H-1 b ), 2.02(m, 1H, H-24 b ), 1.90 (td...
Embodiment 2
[0031] 0.5g (1.2mmol) 3-carbonyl-Δ 4 - Dissolve veratramine (B) in 50mL diethylene glycol, add 1.0mL (20mmol) hydrazine hydrate, 1.2g (21mmol) potassium hydroxide, stir at 160°C, cool down to 120°C after 2h, remove The water in the system was heated up to 180°C and reacted for about 4 hours. The reaction solution was added to 150 mL of ice water, left to stand in the refrigerator for 2 h, and filtered to obtain 0.36 g of a white solid. Crystallize with 50 mL of acetone to obtain 0.20 g of white powder. The mother liquor is applied to a silica gel column, and eluted with a gradient petroleum ether: ethyl acetate mixture to obtain a white solid 3-deoxy-Δ 4 - Veratramine (C) 46mg, yield 52.2%, ESI-MS: m / z 394[M+H] + .
Embodiment 3
[0033] In a stirred 50mL autoclave, add 3-deoxy-Δ 4 - Veratramine (C) 0.3g, ethanol 20mL, Pd / C 50mg, replace the air in the reactor with hydrogen and pressurize to 350psi, react at room temperature for 6-8h, filter and recover Pd / C, evaporate the solvent to obtain a solid 0.3g, put on a silica gel column, elute with a gradient petroleum ether: ethyl acetate mixture to obtain 256 mg of white solid 3-deoxy-5,6-dihydro-veratramine (D), the yield is 85%, ESI -MS: m / z 396[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com