Anti-venomous-snake PLA2 protein antibodies and application thereof
A PLA2, protein technology, applied in the direction of anti-animal/human immunoglobulin, material testing products, instruments, etc., can solve the problems of failing to identify the snake species that bite, delaying the treatment window, etc., to improve the diagnosis of snakebite The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The invention provides a preparation method of an anti-venomous snake PLA2 protein antibody, comprising:
[0067] Step S01, preparing PLA 2 protein antigen.
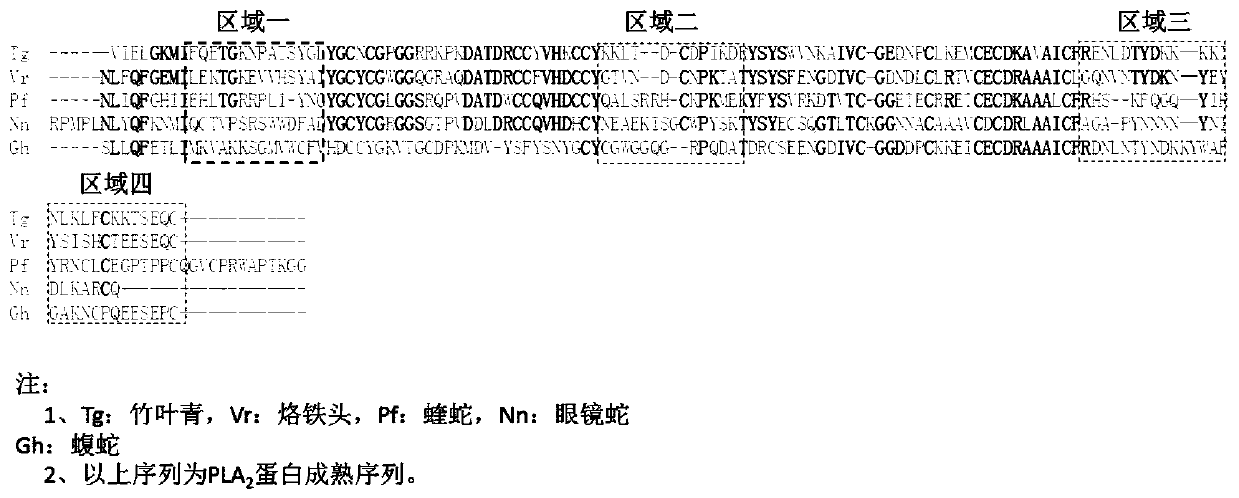
[0068] Specifically, PLA was synthesized by Jill Biochemical (Shanghai) Co., Ltd. through solid-phase synthesis 2 protein peptide antigen. Among them, the amino acid sequences of the ten small peptide antigens are respectively shown in SEQ ID NO: 6-15, and 6 histidines are added to the C-terminus of each small peptide antigen, for example, added to the C-terminus of SEQ ID NO: 6 6 histidines were obtained; 50 mg of polypeptide antigens were synthesized from each short peptide antigen and the purity was greater than 95%.
[0069] Step S02, use PLA 2 The mice were immunized with the protein antigen, and the splenocytes of the immunized mice were obtained.
[0070] Specifically, using the PLA prepared in step S01 2 Five Balb / c mice were immunized with protein antigens respectively. Among them, the Balb / c mouse...
Embodiment 2
[0080] Phyllostachys anti-PLA 2 Protein Monoclonal Antibody Tritoxomab-PLA 2 -1. Tritoxomab-PLA 2 -2 with Phyllostachys PLA 2 Protein epitope binding test, specific steps:
[0081] 1) Coating: Zhuyeqing PLA 2 Protein (5 μg / ml) was diluted with coating solution and added to 96-well plate in an amount of 100 μl per well, and 100 μl of coating solution was added to blank wells, and coated overnight at 4°C.
[0082] 2) Washing: Pour off the coating solution in the wells of the plate the next day, wash three times with washing solution, 250 μl per well, 3-5 minutes each time, and pat dry as much as possible.
[0083] 3) Blocking: 200 μl of blocking solution per well, 37° C. for 1 hour. Wash three times afterwards and pat dry.
[0084] 4) Add 100 μl antibody Tritoxomab-PLA to the positive well 2 -1 or antibody Tritoxomab-PLA 2 -2 (5 μg / ml), add 100 μl of blocking solution to negative wells and blank wells, and keep at 37° C. for 1 hour. Wash three times afterwards and pat d...
Embodiment 3
[0096] This embodiment provides a double-antibody sandwich ELISA detection kit, including a PLA-coated 2 Solid phase carrier for protein antibody, enzyme-labeled PLA 2 Protein antibody, diluent, chromogenic substrate, stop solution, sealing membrane, and positive standard. Among them, PLA 2 Antibodies to proteins including anti-Pipaphyllum PLA 2 Protein monoclonal antibody panel, anti-tip PLA 2 Protein monoclonal antibody panel, Anti-Viper PLA 2 Protein monoclonal antibody panel, Anti-COBRA PLA 2 Protein monoclonal antibody panel, Anti-Apis viper PLA 2 Protein monoclonal antibody panel.
[0097] Phyllostachys anti-PLA 2 Protein monoclonal antibody group (Tritoxomab-PLA 2 -1 and Tritoxomab-PLA 2 -2), Tritoxomab-PLA 2 -1 and Tritoxomab-PLA 2 -2 as coating antibody and enzyme-labeled antibody respectively, wherein, Tritoxomab-PLA 2 -2 includes a detectable label, the solid phase carrier is a microwell plate, magnetic particles or a plastic tube, and the enzyme label i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com