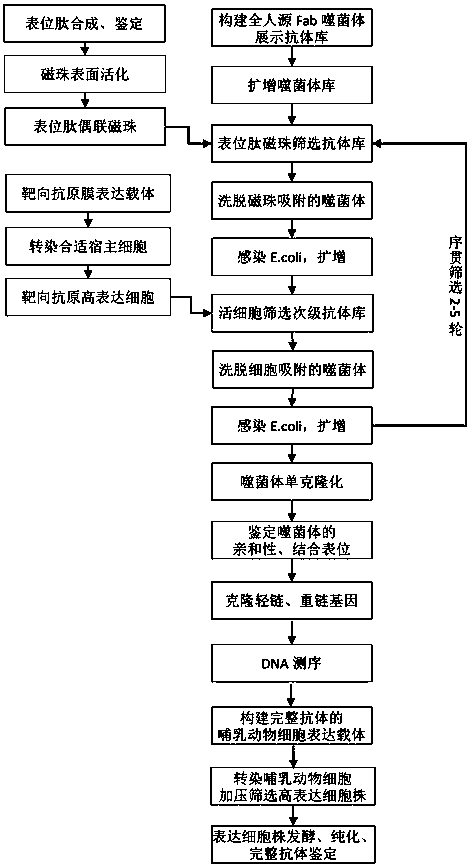
Epitope-specific antibody screening method and screened antibody
A screening method and specific technology, applied in the direction of antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-tumor drugs, etc., can solve the ineffective anti-PD-1 treatment, low antibody hit rate, affinity Advanced problems, to achieve the effect of reducing drug cost and price, high affinity, and improving hit rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. Synthetic PD-1 epitope peptide screening phage display antibody library
[0039] (1) Epitope peptide synthesis and magnetic bead coupling
[0040] According to the sequence of SEQ ID NO: 13, the human PD-1 cyclic epitope peptide was chemically synthesized (synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.). The epitope peptide includes amino acids QTDKLAAF at positions 75-82 of human PD-1 , as well as the cysteine M for magnetic bead coupling and the linking peptide consisting of 3 amino acids on both sides, with a molecular weight of 1475 Da. Dissolve the synthesized epitope peptide in PBS (phosphate buffer, pH7.2, containing 20mM sodium phosphate salt, 150mM sodium chloride) at 10mg / mL for later use;
[0041] Wash 2 mL of amino-derivatized magnetic beads (Thermofisher Scientific company, catalog number 21352, each mL of magnetic beads weighs 2.5 g and contains about 12 µmol amino groups) with 2 mL of PBS, wash three times, and magnetically separa...
Embodiment 2
[0047] Example 2. Secondary screening of functional antibodies by living cells with high expression of PD-1
[0048] (1) Construct a cell line that highly expresses PD-1 on the cell surface
[0049] Clone the full-length PD-1 coding gene (PDCD1 gene, containing the N-terminal signal peptide coding sequence, see NCBIReference Sequence: NM_005018.2 for the specific sequence), insert it into the pcDNA3.1 expression vector, and amplify it in Escherichia coli (E. coli) Transfect 293T cells (ATCC number CRL-3216), screen high expression cell lines, according to the literature "Flow cytometric detection and quantitation of the epidermal growth factor receptor incomparison to Scatchard analysis in human bladder carcinoma cell lines. Cytometry. 1994 Sep 1;17( 1):75-83" and "Antibody-dependent cellularcytotoxicity mediated by cetuximab against lung cancer cell lines. ClinCancer Res. 2007 Mar 1;13(5):1552-61" recorded flow cytometry method to measure the surface of each cell line The ex...
Embodiment 3
[0053] Example 3, Construction of a fully human anti-PD-1 monoclonal antibody expression system
[0054] (1) Identification of heavy and light chain variable regions
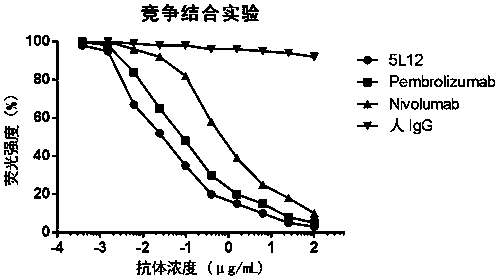
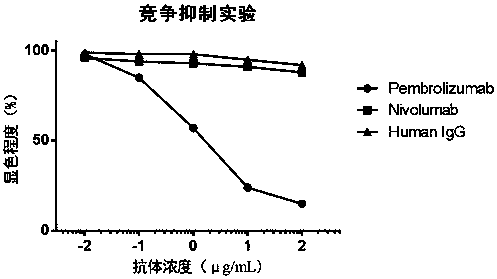
[0055] From the 22 phage-positive clones finally obtained in Example 2, 5 clones with high affinity identified by ELISA were selected for sequencing, and 1 clone numbered 5L12 was selected for further development. Sequencing results showed that the heavy chain variable region of 5L12 had the amino acid sequence of SEQ ID NO: 7, and the light chain variable region had the amino acid sequence of SEQ ID NO: 8. Further antibody sequence analysis showed that the complementarity determining regions of the heavy chain CDR1, CDR2 and CDR3 respectively have the amino acid sequences of SEQ ID NO: 1, SEQ ID NO: 2, and SEQ ID NO: 3, and the complementary determining regions CDR1, CDR2, and CDR3 of the light chain have SEQ ID NO: 4, SEQ ID NO: 5, and SEQ ID NO: 5, respectively. Amino acid sequence of ID NO: 6.
[0056] (2)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com