Human neutralizing antibody or antigen binding fragment thereof and application thereof
A technology for combining fragments and antibodies, applied in the direction of antibodies, applications, carriers, etc., can solve the problem of the decline of neutralizing antibody ability, and achieve the effect of high neutralizing activity and good neutralizing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1: Obtaining antibody sequence information by high-throughput single-cell sequencing
[0126] Blood was collected from patients who recovered after infection with the Delta (B.1.617.2) new coronavirus variant strain, and peripheral blood mononuclear cells (PBMCs) were separated from the fresh blood by gradient centrifugation with Ficoll. And B cells were isolated from PBMC by magnetic bead method, and antigen-binding B cells were enriched. Then, library construction was carried out in the P2 laboratory according to the kit instructions (ChromiumSingle Cell V(D)J Reagent Kits, 10X Genomics). Sequencing was performed on the MGI sequencing platform, running the MGISEQ-2000RS Sequencing Flow cell kit (made by MGI). Data were analyzed after sequencing off the machine, and antibody assembly was performed.
Embodiment 2
[0127] Example 2 Antibody protein expression and purification
[0128] GenScript was entrusted to express and purify the antibody protein.
Embodiment 3
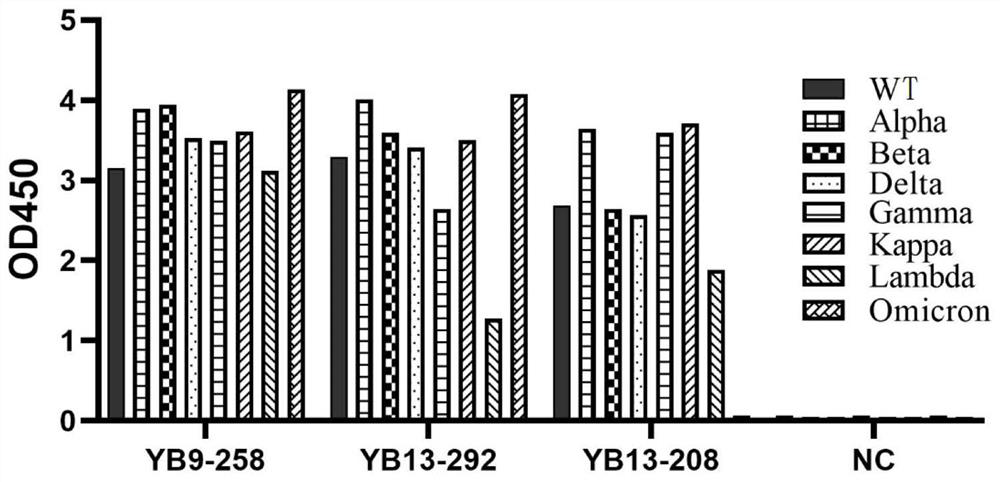
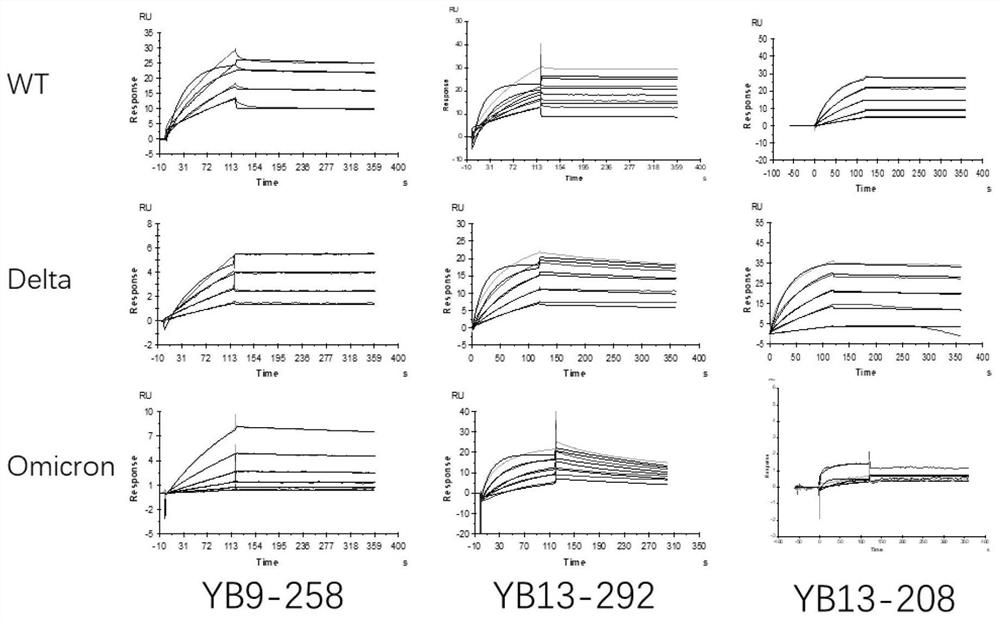
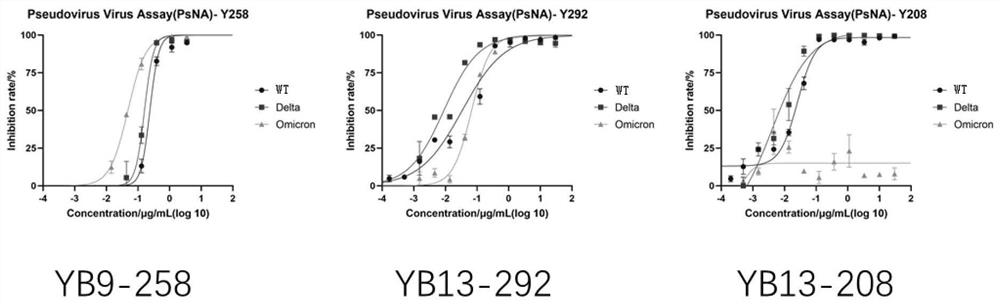
[0129] Example 3 Analysis of the binding ability of neutralizing antibodies to antigens of original and mutant strains of SARS-CoV-2 by ELISA
[0130] Take 50ng of the new coronavirus RBD antigen or the new coronavirus S1 antigen to coat the ELISA plate, 4 ℃, overnight. Washed 3 times with PBST (0.05%). Add 100 ul of 5% nonfat milk powder to each well and block at room temperature for 2 hours. Washed 3 times with PBST. The neutralizing antibody was serially diluted with skim milk powder, the initial concentration was 1.5μg / ml (10nM), and 5-fold serial dilution was carried out in sequence, a total of 8 concentration gradients were added to the wells, each concentration gradient was repeated 3 wells, and 50ul was added. The primary antibody was prepared with skim milk powder at 2ug / ml and incubated at room temperature for 1h. Washed 3 times with PBST. Add 50ul 8000-fold diluted Anti human-FC secondary antibody, incubate at room temperature for 45min, and wash 3 times with PB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com