Anti-h7n9 fully human monoclonal antibody 8d11 and its preparation method and application
A monoclonal antibody, 8D11 technology, applied in the field of immunology, can solve the problems of no effective treatment, drug resistance, etc., and achieve the effect of high affinity and specificity, good biocompatibility, simple and fast operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
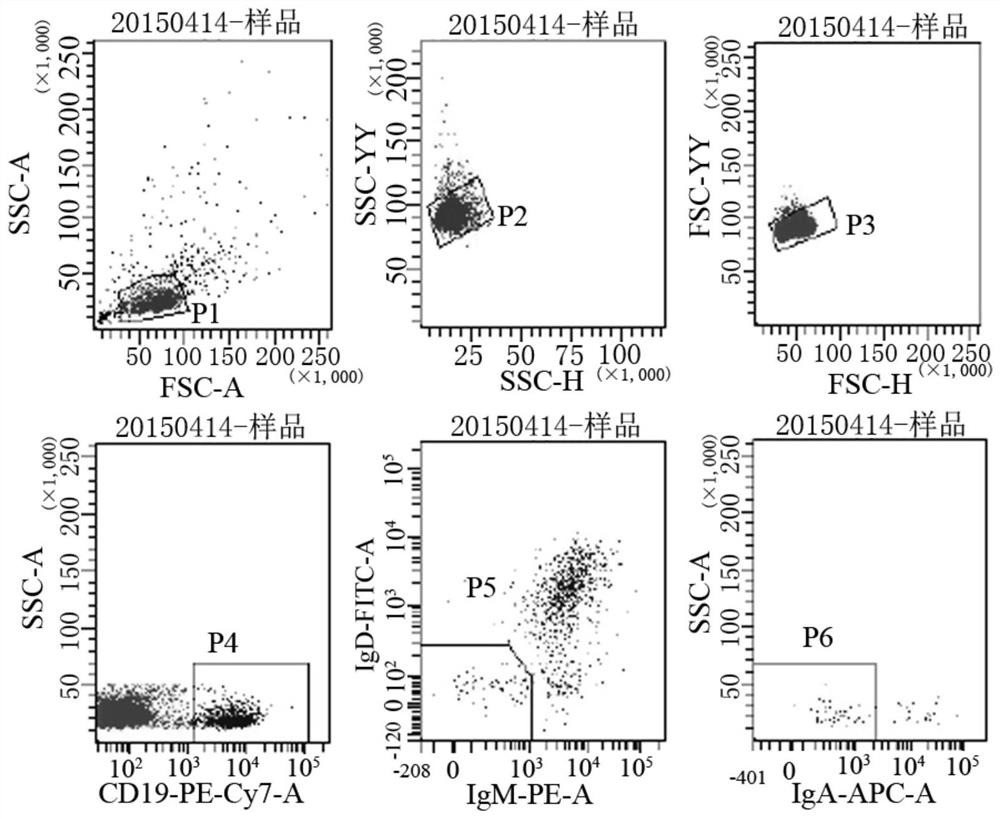
Embodiment 1
[0053] (1) Construction of NTH-3T3 cell line stably expressing CD40L (3T3-CD40L)
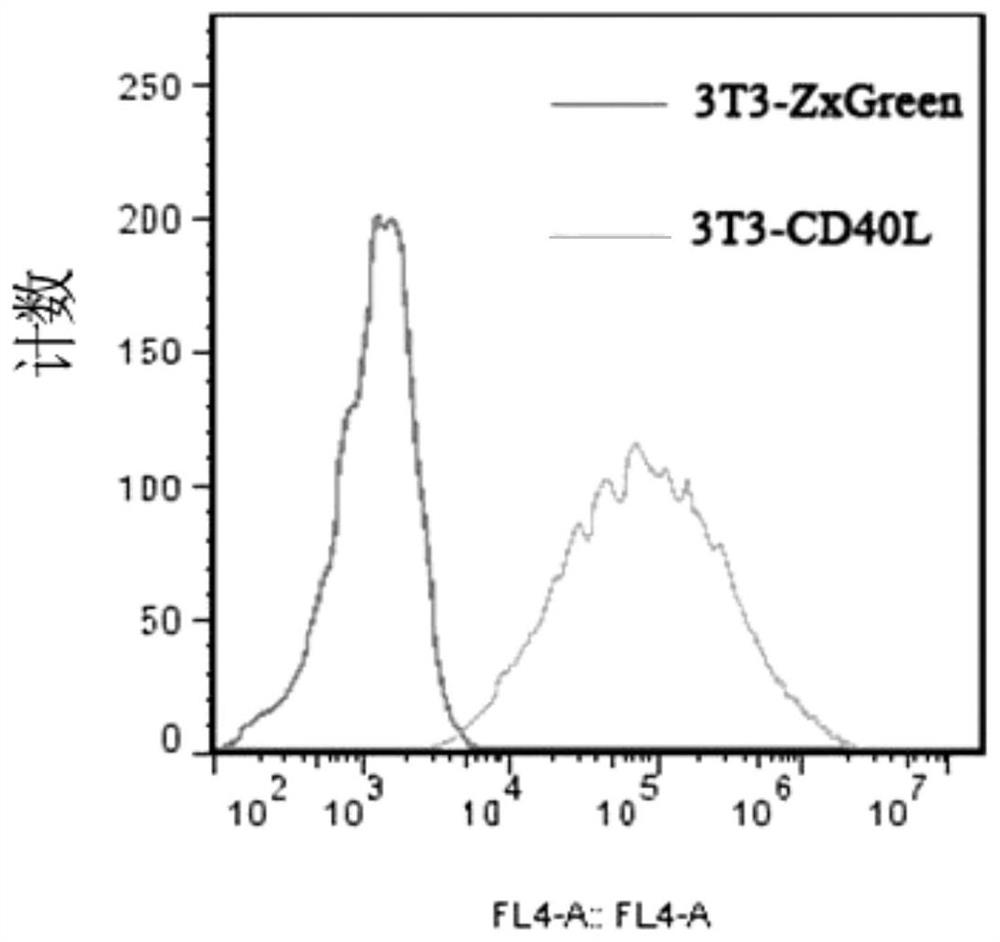
[0054] 3T3-CD40L feeder cells were established using lentivirus. The lentiviral expression vector pLVX-CD40L was constructed and transfected into 293T cells, and the viral supernatant was collected on the fourth day of transfection. NIH-3T3 cells were activated, cultured for 3 passages, infected with lentivirus, and continued to be cultured and passaged 3 times. Cells with FITC fluorescence intensity near MFI were sorted by flow cytometer, added back to the culture flask, 37°C, 5% CO 2 Cultivate and test in an incubator, and the test results are as follows figure 1 As shown, 3T3 cells expressing CD40L and 3T3 cells transfected with empty vector pLVX (with ZxGreen) were stained with anti-CD40L with APC, respectively, and then analyzed by flow cytometry. It was found that all 3T3-CD40L feeder cells expressed CD40L. When the cells grow to 80% to 90%, digest and collect the cells at a concentrat...
Embodiment 2
[0075] Example 2 Cloning, recombination, expression and purification of humanized monoclonal antibody 8D11 gene
[0076] The B cells that can secrete the 8D11 antibody binding to H7N9 virus obtained in Example 1 were lysed, and the lysate was taken for reverse transcription of RNA to obtain the PCR template cDNA of the human antibody gene. Primers for cloning antibody genes were designed and synthesized, genes for heavy and light chains of antibodies were cloned using cDNA as a template, and recombinants were expressed and purified in eukaryotic cells 293F or HEK293. specifically:
[0077] (1) Transfer the lysed B cell solution to a 96-well plate (Eppendorf, 030133366).
[0078] (2) Reverse transcription system: 150ng random primer (Invitrogen, 48190-011), 0.5 μl 10mM dNTP (Invitrogen, 18427-088), 1 μl 0.1M DTT (Invitrogen, 18080-044), 0.5% v / v Igepal CA -630 (Sigma, I3021-50ML), 4U RNAsin (Promega), 6U Prime RNAse Inhibitor (Eppendorf) and 50UIII reverse transcriptase (Invi...
Embodiment 3
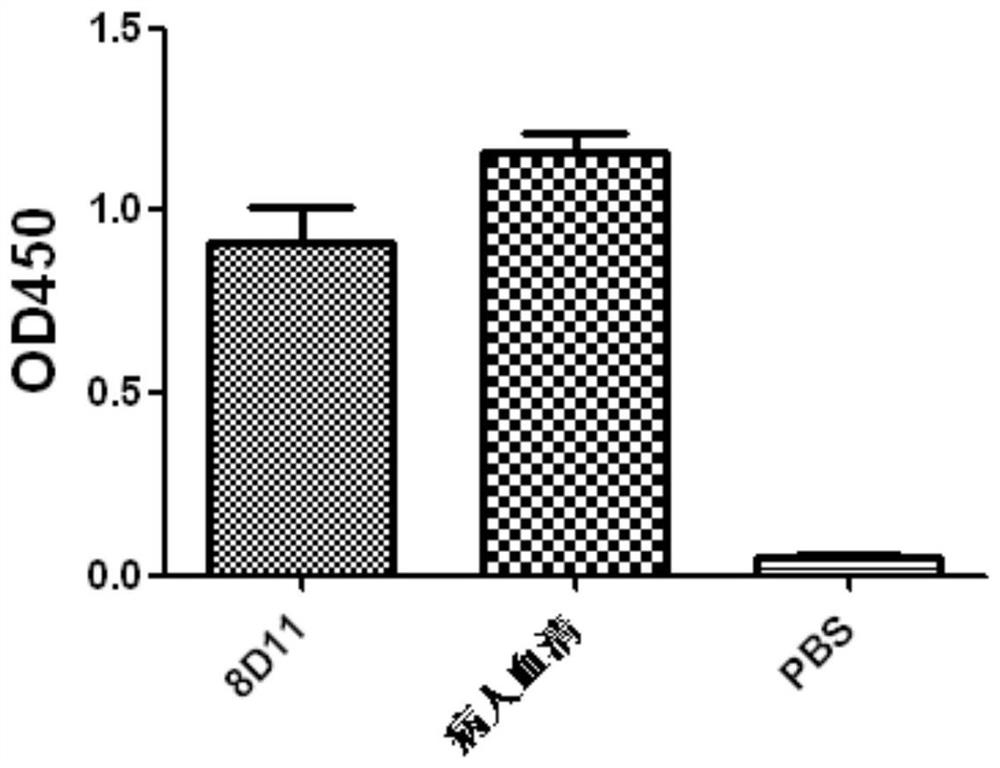
[0132] Example 3 Neutralization test and antibody affinity test of purified fully human monoclonal antibody 8D11
[0133] (1) The purpose of the experiment
[0134] Using a virus-infected cell model (canine kidney cell MDCK), the inhibitory effect and effect of 8D11 antibody on H7N9 influenza virus were evaluated by micro-neutralization-ELISA experiment, and the anti-influenza virus activity of the antibody was detected.
[0135] (2) Experimental steps
[0136] (2.1) Cell plating
[0137] MDCK canine kidney cells in logarithmic growth phase were digested with trypsin, collected by centrifugation after termination, and blown evenly to prepare a single cell suspension; the cell concentration was adjusted to 5×10 with cell culture medium 4 cells / ml, seeded in 96-well cell culture plates, cells were placed at 37°C, 5% CO 2 Incubate overnight in an incubator.
[0138] (2.2) Pretreatment of 8D11 antibody and H7N9 virus (the virus A / Anhui / 1 / 2013 was taken from the Institute of Mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com