Preparation method and application of nanoparticle for showing hepatitis C virus envelope protein E2
An envelope protein, fusion protein technology, applied in the direction of viruses, viral peptides, antiviral agents, etc., can solve problems such as reducing the viral load of secondary infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] Example 1 sE2-Ferritin can be successfully secreted and expressed in Drosophila S2 cells
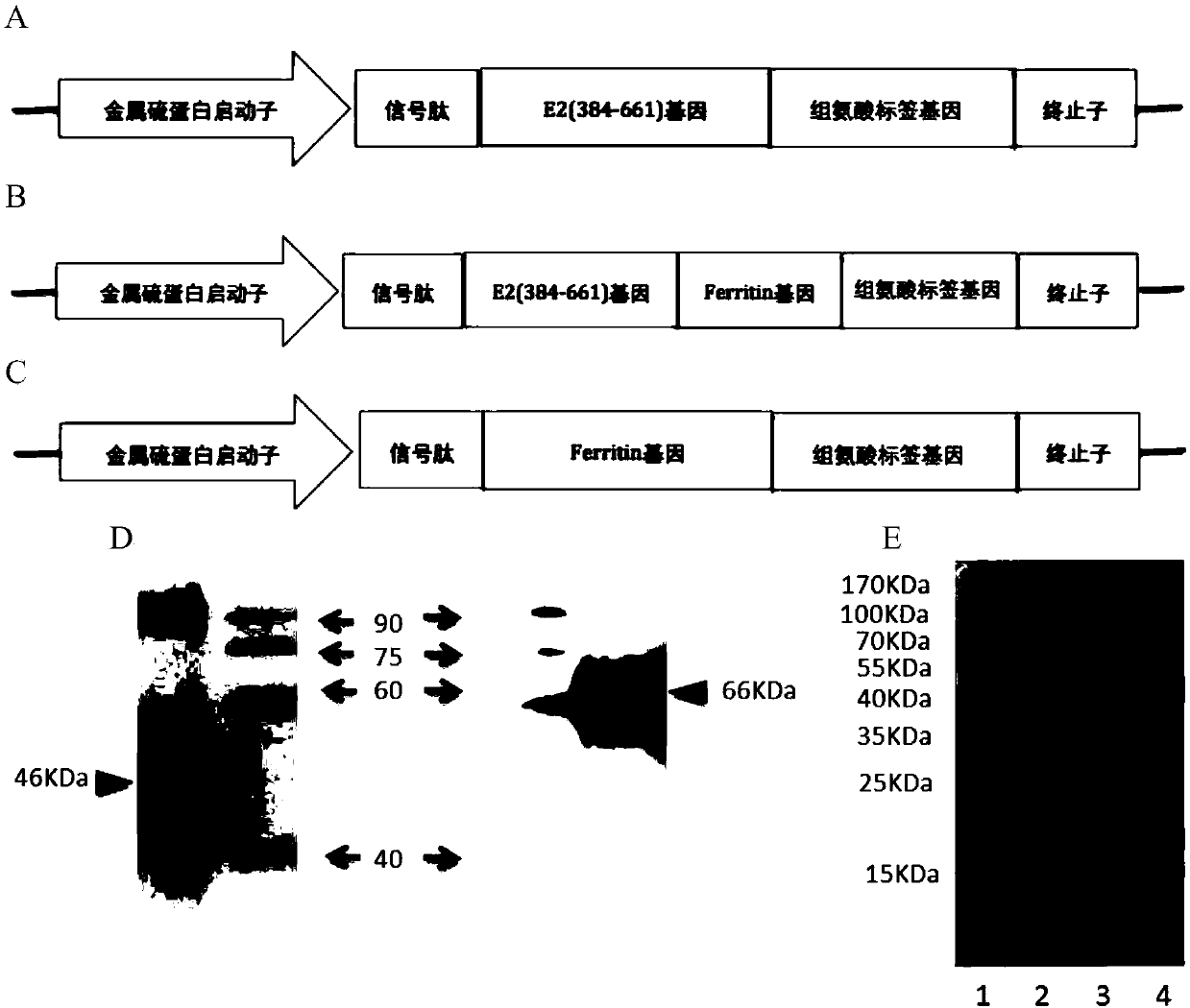
[0165] The recombinant plasmid pMT / Bip / sE2-Ferritin-V5-HisA for secreting and expressing sE2-Ferritin protein in Drosophila S2 cells is as figure 1 As shown in A-C, the truncated secretory segment (384-661) gene of the envelope protein E2 of the standard HCV type 1b strain Con1 was codon-optimized and then linked to the non-heme ferritin carrier gene of Helicobacter pylori for fusion expression, in order to pass the ferritin The HCV envelope protein is correctly displayed on the surface of the ferritin, thereby forming nanoparticles that can self-assemble and secrete in the cell supernatant, and enhance the antigenicity of the envelope protein. In addition, the subunit vaccine recombinant plasmids pMT / Bip / sE2-V5-HisA and pMT / Bip / Ferritin-V5-HisA were used as controls. The sE2-Ferritin and Ferritin gene fragments are followed by a stop codon, while the sE2 protein carries the puri...
Embodiment 2
[0166] Example 2 sE2-Ferritin nanoparticles can be successfully assembled and purified
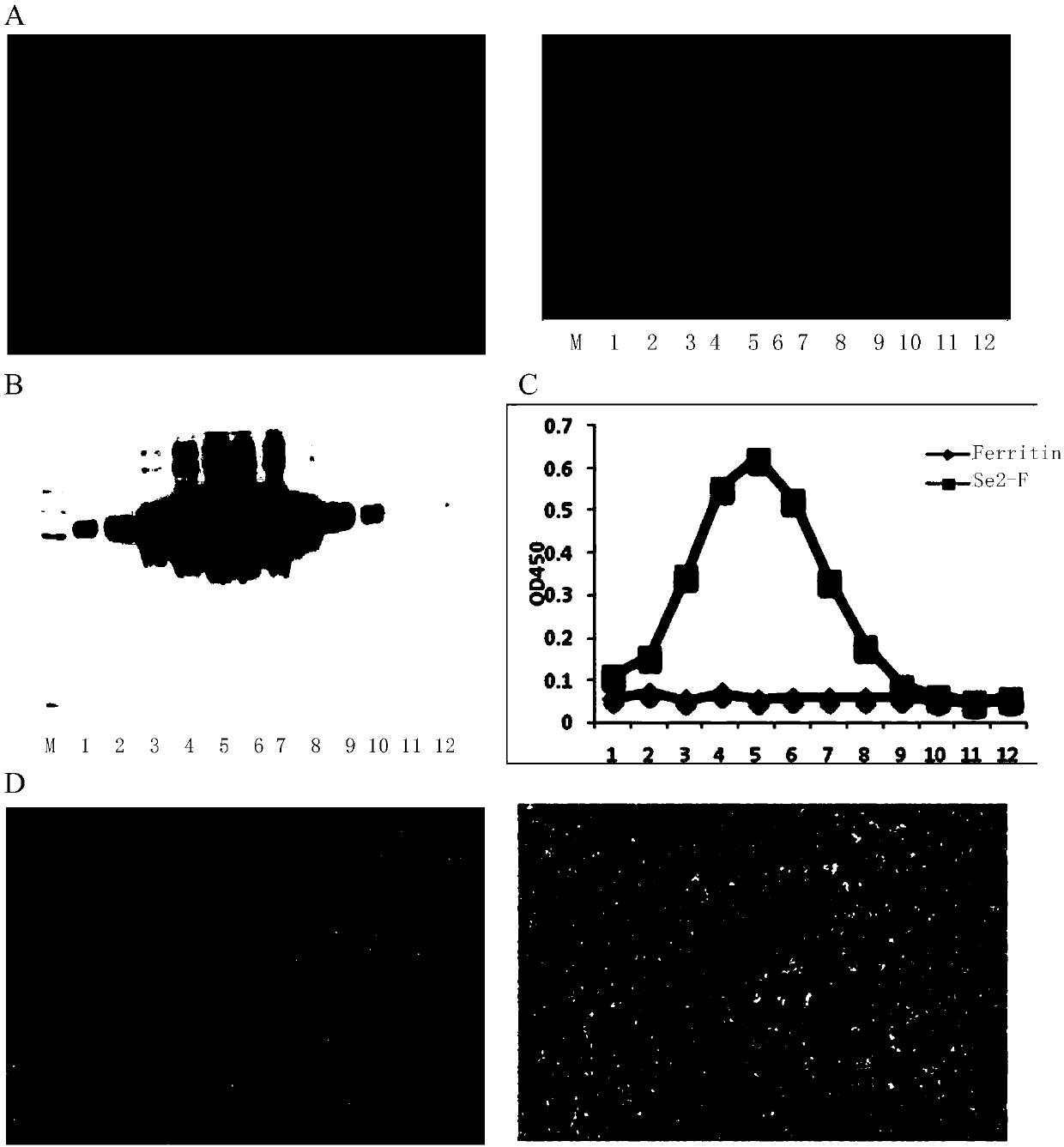
[0167] To determine whether monomeric sE2-Ferritin can self-assemble into nanoparticles. The present inventors purified the protein sE2 secreted into the supernatant as a control through a nickel column. The sE2-Ferritin and Ferritin secreted into the supernatant were passed through a 10% sucrose pad, resuspended in PBS, and then subjected to 10%-50% sucrose density gradient centrifugation, and divided into 12 layers for SDS-PAGE, ELISA and other tests. Depend on figure 2 A Coomassie brilliant blue staining showed that sE2-Ferritin and Ferritin were distributed in layers 5, 6, 7 and layers 3, 4, and 5, respectively. figure 2 B-C showed that the sE2-Ferritin distribution layer detected by immunoblotting and enzyme-linked immunosorbent assay with HCVE2-specific detection antibody 1C9 and conformational antibody 1B4 were consistent with the results of Coomassie brilliant blue staining. I...
Embodiment 3
[0168] Example 3 The HCVsE2 envelope protein displayed by nanoparticles is near native conformation
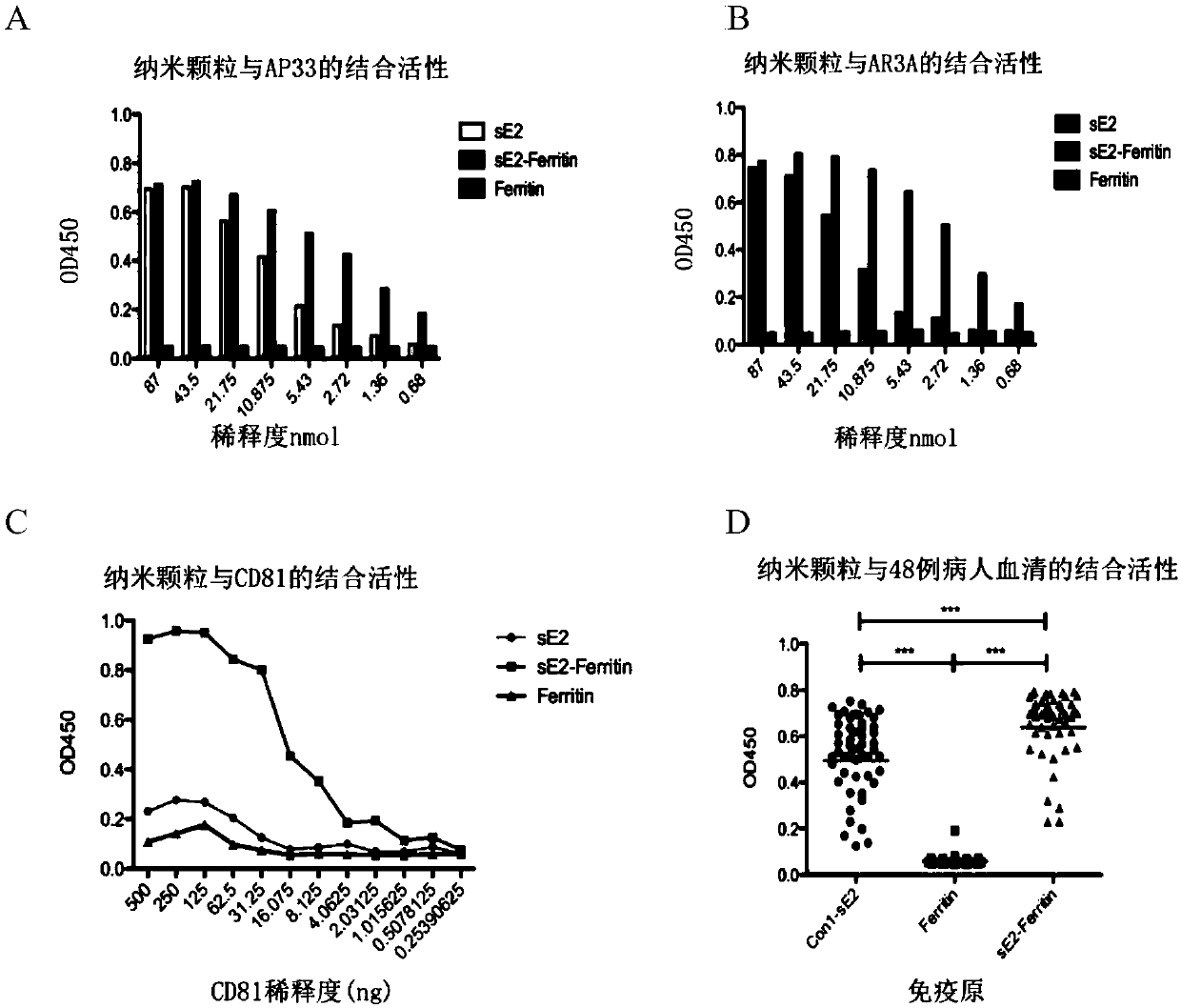
[0169] To test whether the self-assembled nanoparticles displayed sE2 close to the native conformation of HCV envelope protein E2, as image 3 As shown in A-B, the inventors used HCV linear neutralizing antibody AP33 and conformation neutralizing antibody AR3A to bind to sE2-Ferritin nanoparticles, respectively. Both sE2 and sE2 can be combined with both antibodies, and the binding ability of nanoparticles is significantly stronger than that of protein, and the binding is dose-dependent. image 3 C shows that sE2 displayed by nanoparticles can bind to HCV receptor CD81, and the binding is significantly higher than that of sE2 protein in a dose-dependent manner, which indicates that the HCV truncated envelope protein displayed by nanoparticles is close to the native conformation. The binding ability of the conformation neutralizing antibody AR3A to sE2-Ferritin nanoparticles a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com