Gram positive bacteria preparations for the treatment of diseases comprising an immune dysregulation
A technology for autoimmune diseases and preparations, applied in metabolic diseases, skin diseases, sensory diseases, etc., can solve problems such as local ulcers, impossible identification of thermally unstable components, and inability to be used for immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
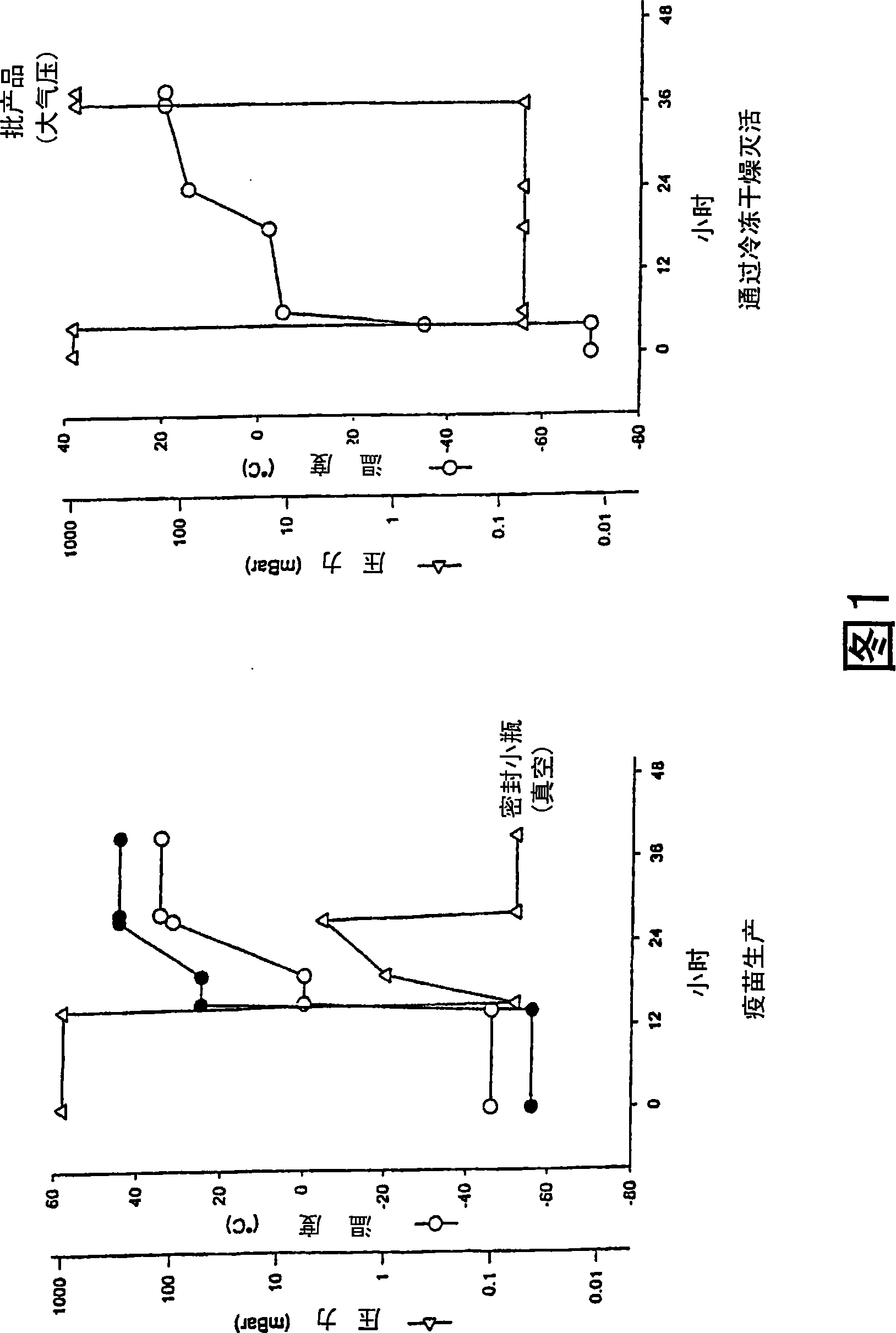
[0133] Example 1: Preparation of deep freeze-dried inactivated mycobacteria
[0134] 1) Materials and methods
[0135] Mycobacterium bovis BCG cells (BCG Pasteur vaccine strain 1173P2, deposited in the French Collection of Microorganisms (CNCM) on April 24, 1978, 25 rue du Docteur Roux, 75724 Paris Cedex 15 (France), deposit number n° I-059 (M. Gheorghiu et al., 1983, Bull. Inst. Pasteur, 81, 281-288) was grown in sterile Sauton medium (HOOCCH (NH 2 O)CH 2 CONH 2 H 2 O (Asparagine), 4g / l; C 6 H 8 O 7 ; H 2 O (citric acid), 2g / l; K 2 HPO 4 (Dipotassium hydrogen phosphate), 0.5g / l; MgSO 4 -H 2 O (magnesium sulfate), 0.50g / l; iron III citrate, 0.05g / l; glycerin, 60ml; zinc sulfate solution (0.155g zinc sulfate dissolved in 10ml pyrogen-free water), 240μl / l, pH 7 ). More specifically, the 1173P2 strain was grown in a 250ml spherical culture flask containing 130ml of sterile Sauton medium and cultured at 37°C for 14 days, which is equivalent to the culture reaching the end of the logar...
Embodiment 2
[0150] Example 2: Preparation of deep freeze-dried inactivated mycobacterial components
[0151] 1) Delipidated fraction (Component A)
[0152] About 10mg / ml of 10 10 Deeply freeze-dried inactivated BCG cells suspended in boric acid buffer (Na 2 B 4 O 7 10H 2 O 0.363%; H 3 BO 3 0.525%; NaCl 0.619% and Tween 200.0005%, dissolved in distilled water, pH 8), centrifuge at 12000g for 10 minutes, discard the supernatant. The precipitate was resuspended in 1ml of chloroform / methanol (9 / 1) and reacted at room temperature for 24h to extract lipids. Centrifuge at 12000g for 10 min to remove chloroform / methanol. The lipid-free precipitate from the chloroform / methanol extract was dried in vacuo.
[0153] 2) Deglycosylated fraction (Component B)
[0154] About 10mg / ml of 10 10 Deeply freeze-dried inactivated BCG cells suspended in boric acid buffer (Na 2 B 4 O 7 10H 2 O 0.363%; H 3 BO 3 0.525%; NaCl 0.619% and Tween 200.0005% were dissolved in distilled water, pH 8), centrifuged at 12000g fo...
Embodiment 3
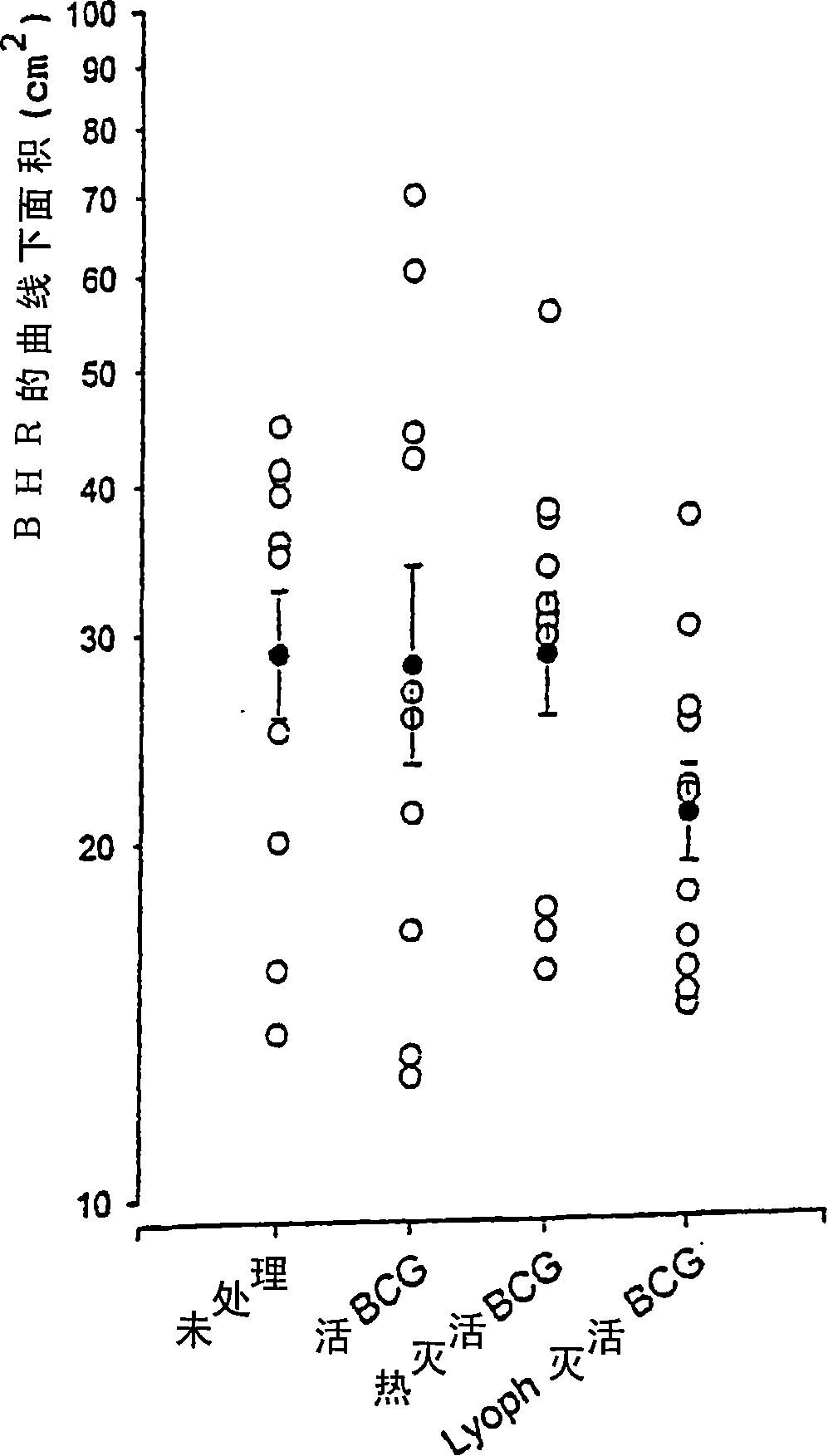
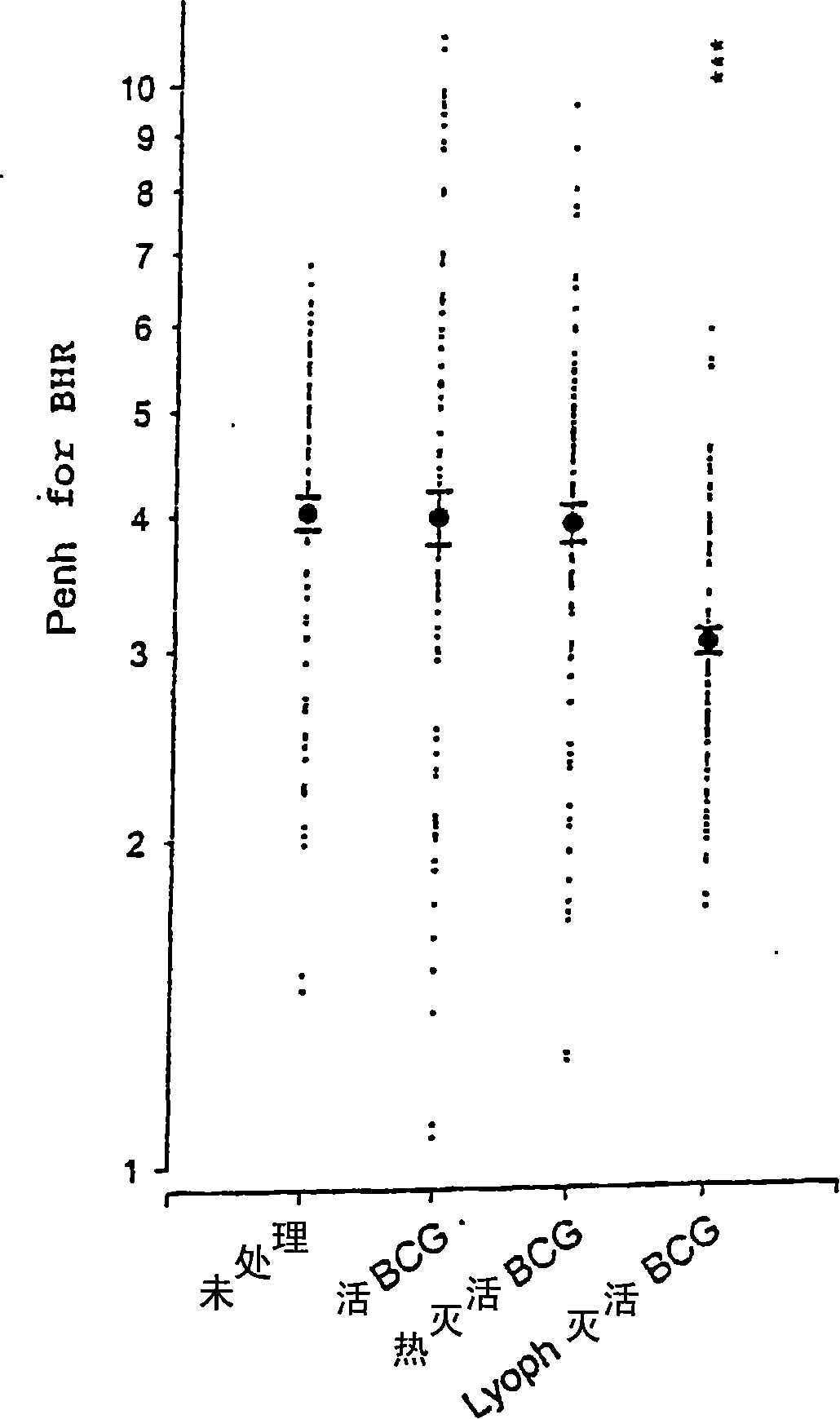
[0171] Example 3: The protective effect of deep freeze-dried inactivated mycobacteria on asthma in a mouse model
[0172] 1) Materials and methods
[0173] a) Animals
[0174] Male mature (6 to 7 weeks old) BP2 (H-2 q ) The mice were from Centre d'élevage R. Janvier (Le Genest, Saint Isle, France) and were raised in an animal breeding facility free of specific pathogens.
[0175] b) Mouse model of asthma-like immunogen-specific disease
[0176] On day 0 (D 0 ) And the 7th day (D 7 ), with 1 μg ovalbumin (OVA; ICN Laboratories) and 1.6 mg aluminum hydroxide adjuvant (dissolved in saline, final volume 0.4 ml), male mature BP2 mice were immunized subcutaneously (neck dorsal side). An intranasal challenge experiment was performed with 10 μg OVA dissolved in 50 μl saline on day 96-98 to induce allergic reactions. Alternatively, a water-soluble ray-grass pollen extract is used as the immunogen, and the immunization is added the same as OVA, but the amount of the extract is 10 μg.
[0177] c)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com