Whole-cell tumor vaccine scaffold and preparation method thereof
A tumor vaccine and whole-cell technology, applied in the field of whole-cell tumor vaccine scaffolds and its preparation, can solve problems such as complicated processes, and achieve the effects of easy acquisition, improved efficiency, and strong antigen immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0020] Example 1 4T1 whole cell tumor vaccine scaffold
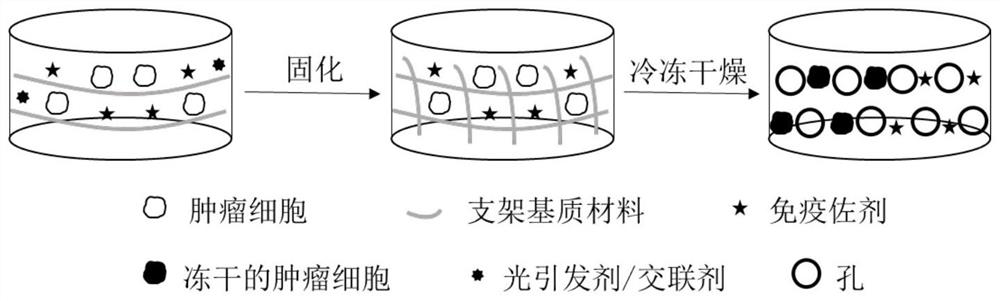
[0021] Preparation: as figure 1 As shown, gelatin-methyl methacrylate was dissolved in deionized water at 60°C, completely dissolved and cooled to room temperature. 200 μL of gelatin-methyl methacrylate solution (30% w / v, containing LAP 0.1% w / w), 50 μL of imiquimod solution (240 μg / mL) and 50 μL of mouse breast cancer 4T1 cell solution (4× 10 7 cells / mL) were mixed evenly, poured into a mold, gelatinized after 405 nm light, placed in a -80°C refrigerator for 2 hours, and freeze-dried to obtain a 4T1 whole-cell tumor vaccine scaffold.
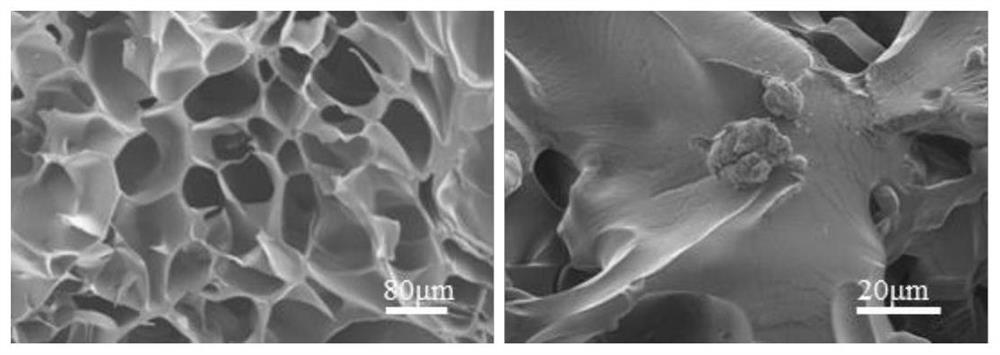
[0022] Characterization: The morphology of the 4T1 whole cell tumor vaccine scaffold was observed under the field emission scanning electron microscope, such as figure 2 As shown, macroporous 3D structures and tumor cells are visible in the scaffolds.
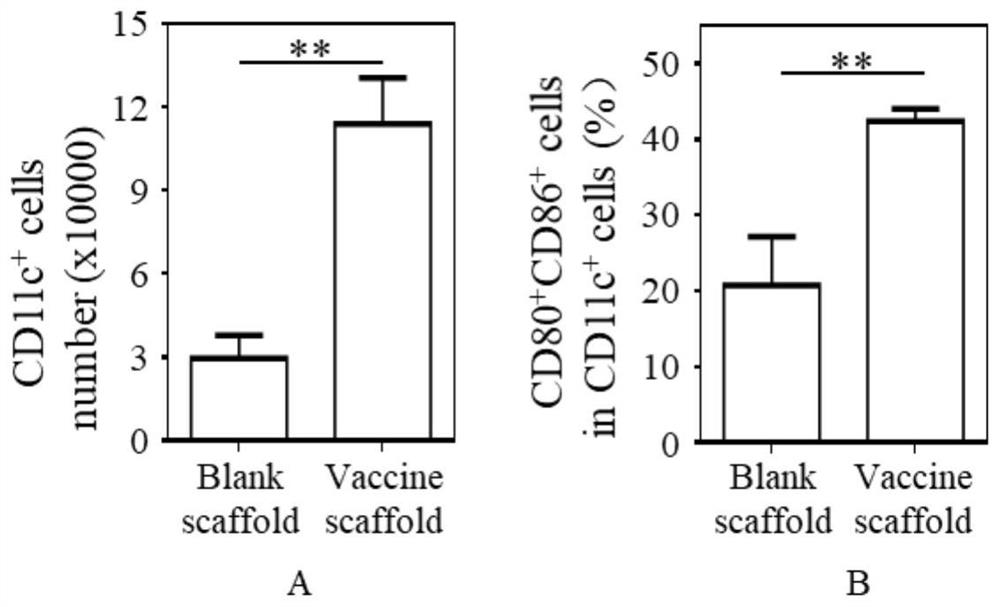
[0023] Recruitment and maturation of dendritic cells: 4T1 whole cell tumor vaccine scaffolds and blank scaffolds were implanted into...
Embodiment 2
[0025] Example 2CT26 whole cell tumor vaccine scaffold
[0026] Preparation: Dissolve gelatin-methyl methacrylate in deionized water at 60°C, cool to room temperature after complete dissolution. Formulated Gelatin-methyl methacrylate (10% w / v), hyaluronic acid-methyl methacrylate (10% w / v), HMPP (1% v / v), monophosphoryl lipid A (400 μg / mL), mouse colon cancer CT26 cells (1 × 10 8 cells / mL) mixed solution, poured 300 μL of the mixed solution into a mold, irradiated with ultraviolet light to form a gel, placed in a -20°C refrigerator for 2 hours, and then freeze-dried to obtain a CT26 whole-cell tumor vaccine scaffold.
Embodiment 3
[0027] Example 3 HepG2 whole cell tumor vaccine scaffold
[0028] Chitosan was dissolved in a 0.5% acetic acid solution to obtain a chitosan solution with a concentration of 2wt%, and sodium glycerophosphate was added to adjust the pH value. The gelatin was dissolved in distilled water to obtain a gelatin solution with a solubility of 4 wt%. The gelatin and chitosan were thoroughly mixed 1:1 (v / v). Genipin (0.5w / w), human hepatoma cell HepG2 (1×10 8 cells / mL), CpG oligodeoxynucleotides (250μg / mL) were added to the mixed solution of gelatin and chitosan, 400 μL of the mixed solution was poured into the mold, placed at 37°C to form a gel, and placed in a -80°C refrigerator After 2 hours, the HepG2 whole cell tumor vaccine scaffold was obtained by freeze-drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com