An oral drug delivery system using composite nanomaterials as a carrier
A technology of composite nanomaterials and drug delivery systems, applied in the oral Helicobacter pylori subunit vaccine, HP55/PBCA nanoparticle preparation field, can solve the problem of improving the bioavailability of oral protein drugs and resistance to gastrointestinal proteases Hydrolysis is weak and other problems, to achieve the effect of enhancing local mucosal immunity, preventing and treating Helicobacter pylori infection, and the preparation method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
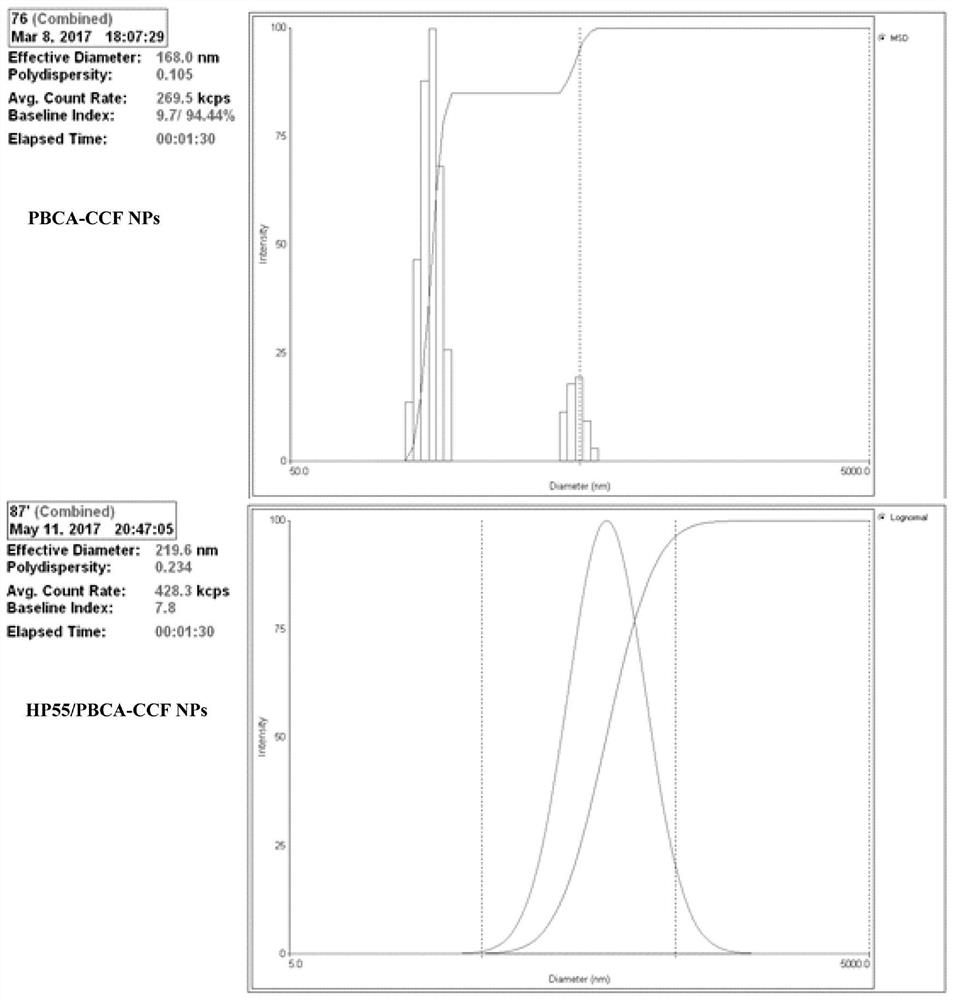
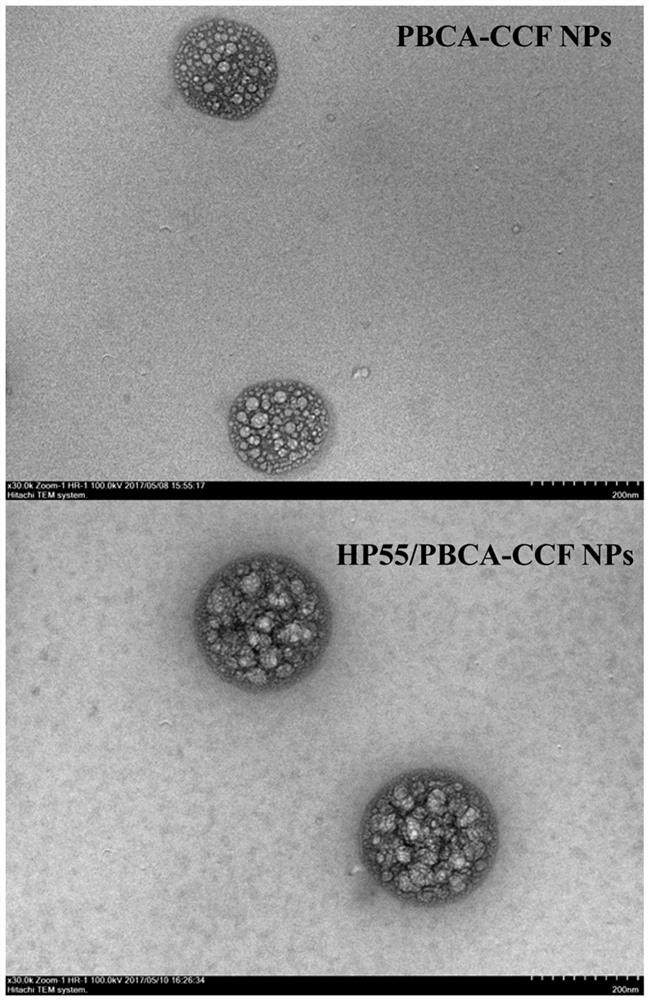
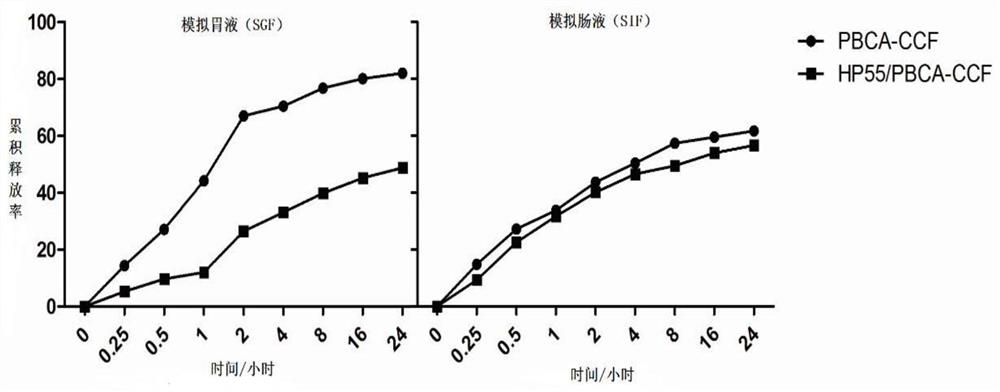
[0046] Example 1: Preparation of PBCA-CCF NPs and HP55 / PBCA-CCF NPs.
[0047] (1) Weigh the prescribed amount of 25 mg HP55 and absorb 50 μL of BCA monomer, dissolve in 5 mL of acetone, and prepare the drug-loaded organic phase for use.
[0048] (2) Weigh 50 mg of poloxamer 188 and dissolve it in 5 mL of the antigenic protein solution separated and purified in our laboratory to make an aqueous phase solution.
[0049] (3) Add the organic phase prepared in step (1) dropwise into the aqueous phase prepared in step (2) with a syringe under 350rpm magnetic stirring at 4°C, and continue stirring for 4.5 hours after the addition is complete , the acetone was evaporated and removed, and finally filtered with a 0.45 μm filter membrane to obtain a milky white stable and uniform HP55 / PBCA nanoparticle suspension.
[0050] (4) Put the nanoparticle suspension into a suitable container and pre-freeze at -20°C for 12 hours, then freeze-dry and store.
[0051] Using the same method and the...
Embodiment 2
[0054] Example 2: PBCA-CCF NPs and HP55 / PBCA-CCF NPs vaccines induce a high level of systemic immune response.
[0055] (1) Grouping scheme of SPF BALB / c mice: (A) NC group: Mice were orally administered a suspension of 500 μL PBS and aluminum hydroxide adjuvant. (B) HP55 / PBCA-group: Mice were orally administered composite HP55 / PBCA nanoparticles without antigen encapsulation. (C) HP55 / PBCA-CCF group: Mice were orally administered composite HP55 / PBCA nanoparticles encapsulated with 100 μg CCF. (D) PBCA-CCF group: Mice were orally administered ordinary PBCA nanoparticles encapsulated with 100 μg CCF. (E) Alum-CCF group: Mice were orally administered a suspension of 100 μg CCF and aluminum hydroxide adjuvant.
[0056] (2) Mice immunization and challenge program: according to Figure 4 , orally immunized 4 times by intragastric administration, with an interval of 7 days each time, and challenged the immunized mice once two weeks later: intragastric administration of Hp suspens...
Embodiment 3
[0062] Example 3: PBCA-CCF NPs and HP55 / PBCA-CCF NPs vaccines induce secretory IgA.
[0063] H. pylori can selectively colonize the gastric mucosa and, if not cleared, will cause chronic inflammation. Secretory IgA is a marker of local mucosal immune response, and the detection of gastric sIgA has guiding significance for judging the preventive and therapeutic effect of vaccines.
[0064] Gastric mucosa sample collection protocol: according to Figure 4 , 4 weeks after the last immunization, the mice were sacrificed by intraperitoneal injection of excess ether, the stomach of the mouse was taken, and the stomach was cut longitudinally along the greater curvature of the stomach, and 0.1 g of gastric tissue was taken, added to 500 μL of PBS, and fully homogenized with a homogenizer , centrifuged at 3000rpm for 30min, and collected the supernatant.
[0065] In order to study the level of gastric mucosal immune response, we used ELISA method to detect the level of sIgA in the st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com