New production process for large-scale synthesis of long-chain RNA drugs
A large-scale, new process technology, applied in the field of long-chain RNA drugs, can solve the problem of no solution for DNA template protein residues, unsolved separation of long-chain RNA complete transcripts, RNA transcripts, and RNA products that cannot meet the needs of RNA drug development, etc. problems, to achieve a reliable and stable synthesis method, reduced host DNA contamination, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Transcription synthesis and purification of miRNA precursor
[0055] 1. Reagents, materials and instruments
[0056] 1. Reagent materials: Pfu DNA Polymerase (purchased from Biomics Biotechnology Co., Ltd.), T7 RNA polymerase (endotoxin content is less than 10EU / mg, purchased from Biomics Biotechnology Co., Ltd.), RNase H-free 2 O, 70% ethanol aqueous solution (RNase-free), rNTP (100mM) (purchased from Biomics Biotechnology Co., Ltd., unmodified), isopropanol, 10×Reaction Buffer (PCR & Transcription, purchased from Biometics Biotechnology Co., Ltd.), Buffer A (5mmol / L EDTA, 5mmol / L Tris-HCl, pH7.5), Buffer B (1M NaCl, 5mmol / L EDTA, 5mmol / L Tris-HCl, pH7.5). All chemical reagents were of analytical grade and were purchased from Shanghai Sangon Bioengineering Co., Ltd. unless otherwise specified.
[0057] 2. Instruments: High pressure liquid chromatography system (Shimadzu LC-20AT), HPLC separation column HR16-Source 15Q (General Medical GE); nucleic acid anal...
Embodiment 2
[0102] Example 2 Preparation of DNA transcription template by PCR amplification and transcription synthesis of miRNA precursor
[0103] 1. Experimental materials: the same as in Example 1.
[0104] 2. Experimental steps:
[0105] 1) Design a pair of primers (chemically synthesized by Biomaike Biotechnology Co., Ltd.)
[0106] hsa-mir-21-F: 5'-CACTAATACGACTCACTATAGG-3' (SEQ ID NO: 6);
[0107] hsa-mir-21-R: 5'-TGTCAGACGACCCATCGACT-3' (SEQ ID NO: 7).
[0108] 2) Template preparation and purification
[0109] Using the miRNA precursor (has-miR-21) DNA transcription template obtained in Example 1 as a PCR template, follow the conventional PCR program (95°C 3min; 95°C 30sec, 55~68°C 30sec, 72°C 1min, 25Cycles; 72°C 5min) for PCR amplification, reaction system: DNA template (2.5ng / μl) 1μl, hsa-miR-21-F (10μM) 2μl, hsa-miR-21-R (10μM) 2μl, 10×PCR Buffer 5μl, dNTPs (2.5mM) 4μl, Taq DNA polymerase (2.5U / μl) 0.5μl, and ultrapure water to make up to 50μlL.
[0110] Template purific...
Embodiment 3
[0114] Example 3 Synthesis and purification of one-strand multi-target siRNA
[0115] 1. Experimental material: with embodiment 1.
[0116] 2. Experimental steps:
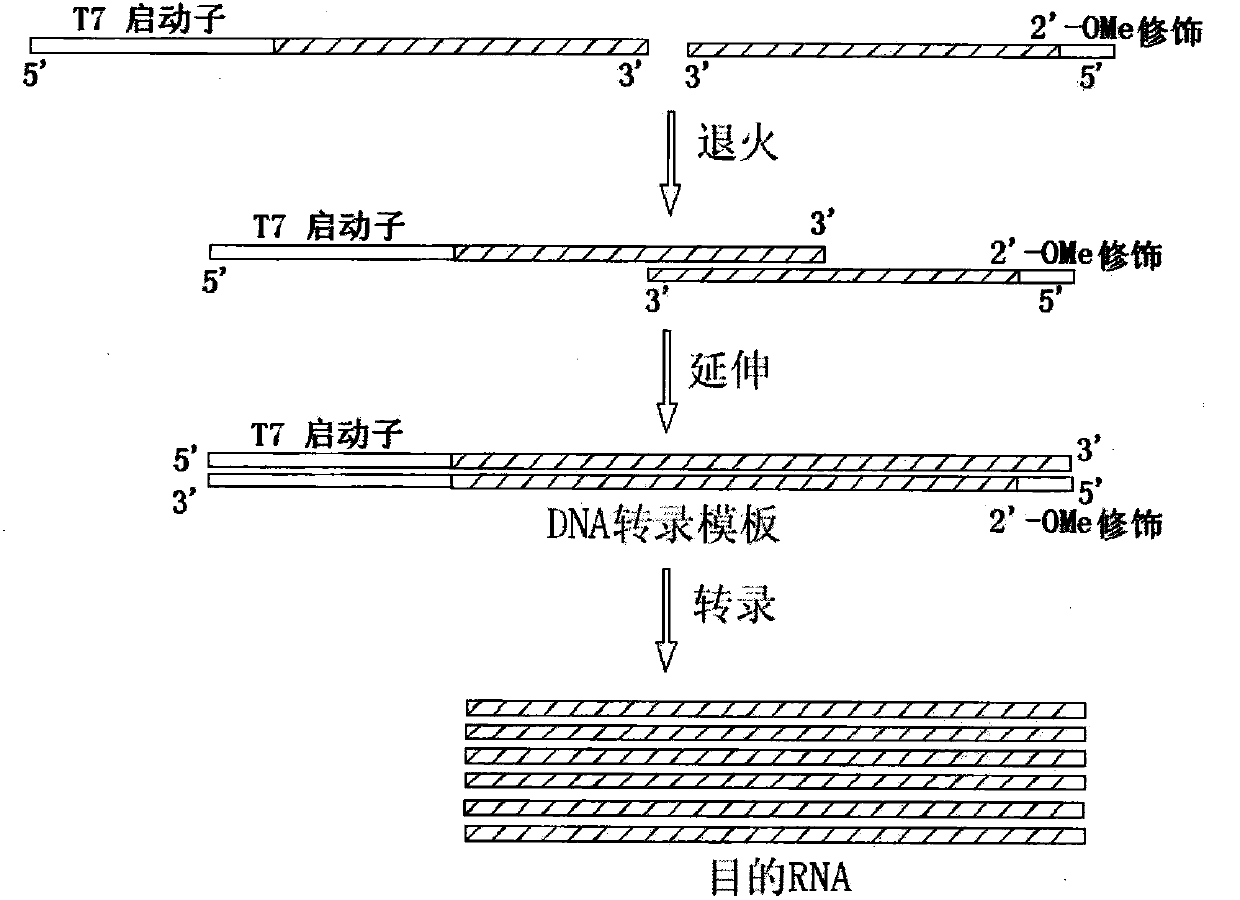
[0117] 1) Sequence design: one-strand multi-target siRNA (SUB) sequence, DNA transcription template sequence, DNA primer sequence design such as Figure 10 shown. Design T-SUB and T-SUB XS DNA transcription templates with different SUB sense strand RNAs. The difference between the two is that the first two bases at the 5' end of the antisense strand of the transcription template are modified with 2'-OMe, and the unmodified DNA transcription template is named For T-SUB, the DNA transcription template modified with 2'-OMe at the 5' end is named T-SUB XS. DNA primers were chemically synthesized by Biomaike Biotechnology Co., Ltd.
[0118] 2) Preparation and purification of transcription template and in vitro transcription reaction: the proportions of the components in the reaction system were the same as those in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com