Bacterial polysaccharide protein conjugate vaccine using hepatitis B surface antigen as carrier protein and preparation method of bacterial polysaccharide protein conjugate vaccine
A technology of hepatitis B surface antigen and bacterial polysaccharide, which is applied in the field of human vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 7:- 2 preparation 。 Embodiment 8(1)~(13):- preparation ; Embodiment 9(1)~(13): preparation 。 Embodiment 10: test 。 Embodiment 11(1)~(4): test 。 Embodiment 12
[0054] Embodiment 1~embodiment 5 is the preparation of bacterial polysaccharide. Example 6: Preparation of hepatitis B surface antigen. Example 7: Preparation of bacterial polysaccharide-adipic hydrazide derivatives. Embodiment 8 (1)-(13): Preparation of bacterial polysaccharide-hepatitis B surface antigen conjugate stock solution; Embodiment 9 (1)-(13): Preparation of vaccine product. Example 10: Effectiveness test of vaccine products. Example 11(1)-(4): Safety test of vaccine products. Embodiment 12: Statistical analysis of test results.
Embodiment 1
[0055] Example 1 Preparation of Group B streptococcus and Streptococcus pneumoniae polysaccharide
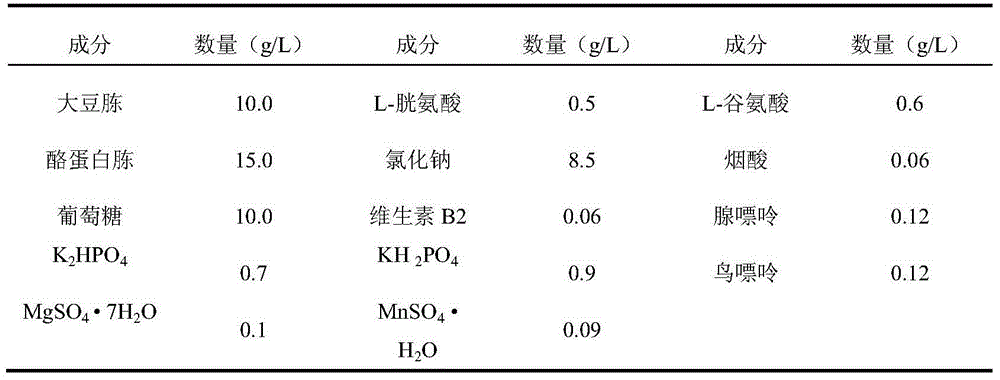
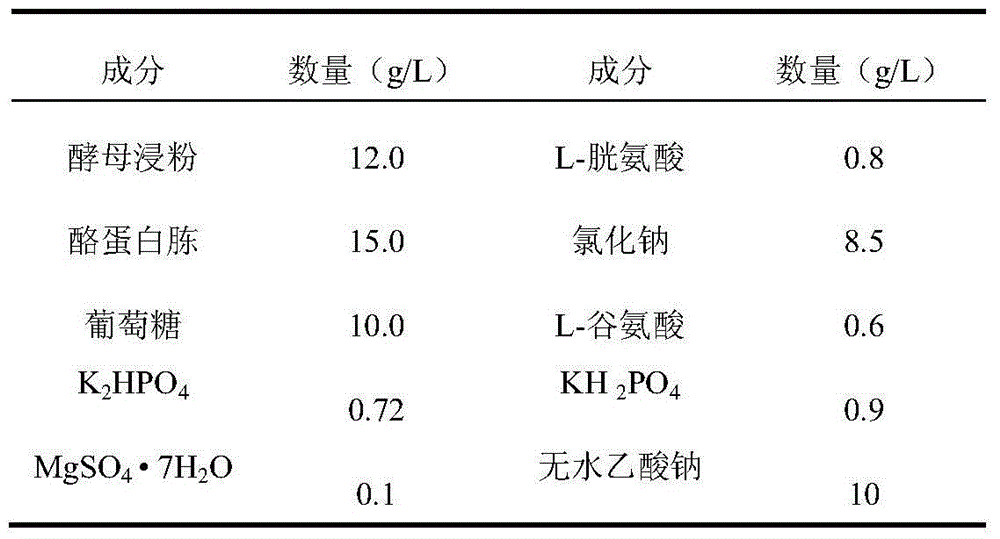
[0056] (1) Fermentation: Use Type Ia Group B Streptococcus, Type Ib Group B Streptococcus, Type II Group B Streptococcus, Type III Group B Streptococcus, Type V Group B Streptococcus, Type 1 Pneumococcus, Type 2 Pneumococcus Pneumococcus type 3, pneumococcus type 4, pneumococcus type 5, pneumococcus type 6A, pneumococcus type 6B, pneumococcus type 7F, pneumococcus type 8, pneumococcus type 9N, pneumococcus type 9V, pneumococcus type 10A Pneumococcus type 11A, pneumococcus type 14, pneumococcus type 15B, pneumococcus type 17F, pneumococcus type 18C, pneumococcus type 19A, pneumococcus type 19F, pneumococcus type 20, pneumococcus type 22F, pneumococcus type 23F The working seeds of coccus or 33F pneumococcus were respectively inoculated in the improved semi-comprehensive medium, and cultured in a carbon dioxide environment at 35-37°C for 4-12 hours, when the culture medium OD 600...
Embodiment 2
[0061] Example 2: Preparation of meningococcal polysaccharide
[0062] The working seeds of Neisserial meningococcus of group A, group C, group Y or group W135 were respectively used to prepare corresponding meningococcal polysaccharides according to the following methods:
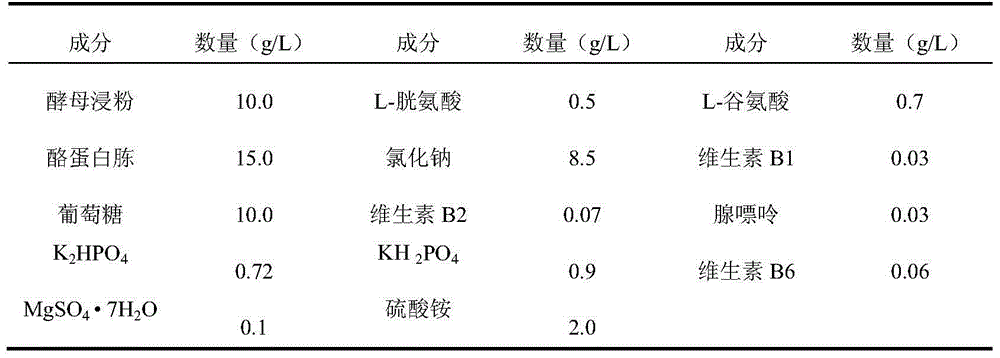
[0063] Inoculate the working seeds of group A or group C or group Y or W135 Neisserial meningococcus on the agar medium containing 10% (volume fraction) sheep blood, and cultivate in a carbon dioxide environment at 35-37°C for 16-20 hours , the Gram staining microscope was qualified, inoculated in a fermenter, and sterilized with formaldehyde solution when cultured in a meningococcal modified semi-comprehensive medium to the late logarithmic growth phase. Add cetyltrimethylammonium bromide to the supernatant collected by centrifugation, and mix the precipitate thoroughly; collect the precipitate by centrifugation, add calcium chloride to dissociate, collect the supernatant by centrifugation, add ethanol un...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com